The hydrogenation of ketones and alkenes has a variety of applications, e.g., in the production of fine chemicals, fragrances, pharmaceuticals, or functional materials. Classical reduction methods can require the use of potentially dangerous hydrogen gas and/or high temperatures and pressures. Environmentally friendly, economical, simple, and safe reduction methods are, thus, interesting research targets.
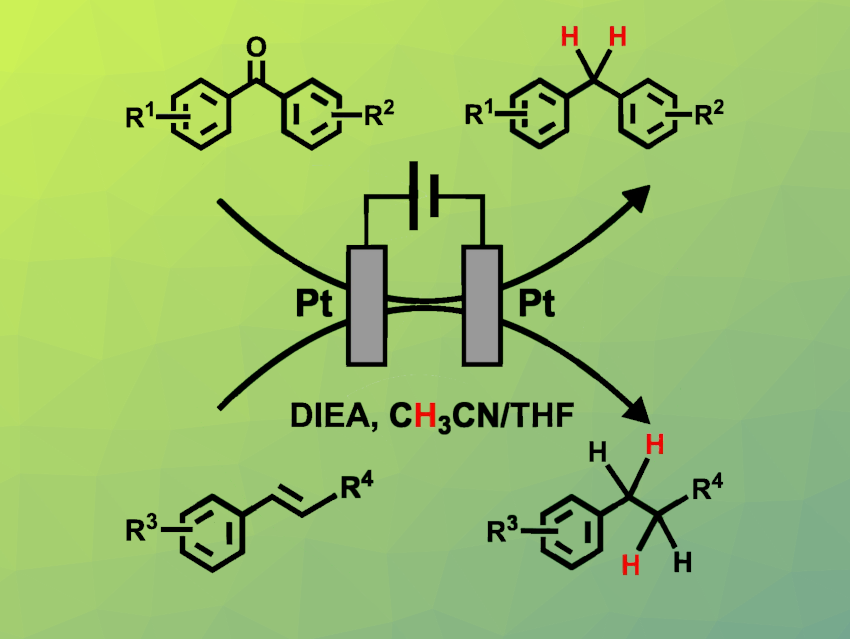
Yahui Zhang, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Guangyan Qing, Dalian Institute of Chemical Physics, Wuhan Textile University, China, and Hubei Jiangxia Laboratory, Wuhan, China, and colleagues have developed a new approach for the electrochemical hydrogenation of C=O and C=C bonds under mild conditions (pictured). The reaction was performed using two platinum plate electrodes in an undivided cell, with tetrabutylammonium tetrafluoroborate (nBu4NBF4) as a supporting electrolyte, N,N-diisopropylethylamine (DIEA) as a sacrificial electron donor, and a mixed solvent system composed of CH3CN and THF (THF = tetrahydrofuran).
Using this approach, a range of diarylketones featuring various substituents on the phenyl rings could be effectively transformed into the respective diarylalkanes. In addition, different aryl alkenes underwent electrochemical hydrogenation to the corresponding alkanes. The team found that CH3CN acts as the main proton donor. The successful completion of gram-scale experiments demonstrates the potential of this strategy for industrial applications.
- Electrochemical Reduction of Diarylketones and Aryl Alkenes,
Ce Bi, Xiangyu Zhao, Zhiqi Jia, Yongxin Chang, Hang Yang, Mengyuan Song, Xin Zhang, Yahui Zhang, Guangyan Qing,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202300258