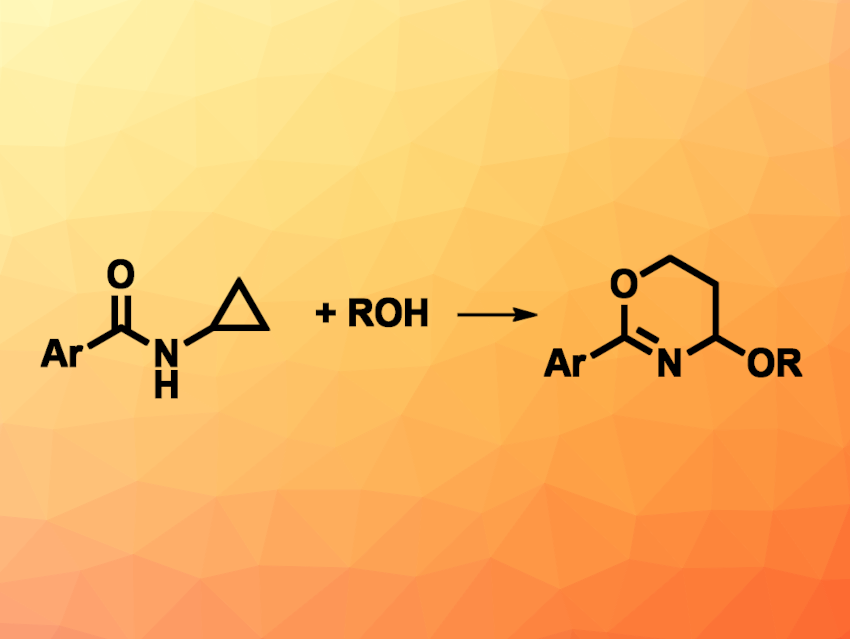
1,3-Oxazines are an important class of heterocycles found, for example, in pharmaceutically active molecules and natural products. New methods for their synthesis are interesting research targets. Cyclopropylamines can be versatile building blocks in organic synthesis, e.g., when used in oxidative ring-opening reactions. A general approach to the synthesis of 1,3-oxazines from such cyclopropylamines had not been realized so far.
Shenlin Huang, Nanjing Forestry University, Jiangsu, China, and colleagues have developed an electrochemical method for the synthesis of 1,3-oxazines via the oxidative ring-opening of cyclopropylamides with alcohols. The team reacted different N-cyclopropylamides with aromatic substituents with a range of different alcohols in an undivided electrochemical cell with a graphite anode and a platinum cathode at a current of 8 mA, using nBu4NBF4 as an electrolyte in the presence of NaI, NaOMe, ferrocene, and 4-dimethylaminopyridine (DMAP), with CH2Cl2 as the solvent.
The desired 1,3-oxazines were obtained in moderate to high yields. The reaction tolerated primary alcohols and secondary alcohols (with moderate yields), and the team found that the tertiary alcohol 1-adamantanol also gave the desired product in 39 % yield. Overall, the method avoids the need for external oxidants and has a broad substrate scope.
- Electrochemical Ring-Opening of Cyclopropylamides with Alcohols toward the Synthesis of 1,3-Oxazines,
Peng-Cheng Xu, Shencheng Qian, Xiangtai Meng, Yu Zheng, Shenlin Huang,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03537