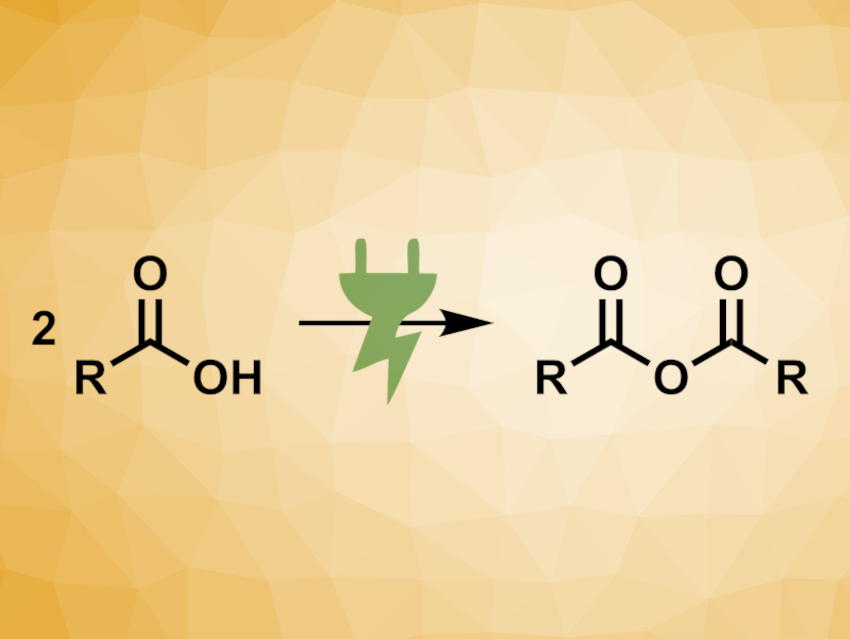
Carboxylic acid anhydrides are versatile reagents in organic synthesis. Anhydrides have some advantages over the analogous carboxylic acid halogenides. For example, acid anhydrides do not produce hydrogen halides as byproducts that could lead to unwanted side reactions. Also, the reactions can often be milder, which allows for more selective transformations. Generally, anhydrides are synthesized from the respective carboxylic acids using stoichiometric amounts of harsh dehydration agents.
Siegfried R. Waldvogel, University of Mainz, Germany, and colleagues have developed an electrochemical synthesis of carboxylic anhydrides from carboxylic acids (pictured). Graphite and stainless steel were used as inexpensive electrode materials for the non-decarboxylative electrolysis of carboxylic acids, using acetonitrile as a solvent and, e.g., KSCN as the supporting electrolyte.
The team converted a broad scope of carboxylic acids to the corresponding anhydrides. The use of electricity allowed them to avoid reagent waste compared with conventional protocols and could provide sustainable access to valuable carboxylic acid anhydrides. Several functional groups, including double bonds and halogen atoms, are tolerated. The electrode materials are low in cost and can be used multiple times without corrosion.
- Beyond Kolbe and Hofer–Moest: Electrochemical Synthesis of Carboxylic Anhydrides from Carboxylic Acids,
Andreas P. Häring, Dennis Pollok, Benjamin R. Strücker, Vincent Kilian, Johannes Schneider, Siegfried R. Waldvogel,
ChemistryOpen 2022.
https://doi.org/10.1002/open.202200059