Dibenzothiophene-based compounds are useful, e.g., in materials and pharmaceutical chemistry. Existing methods for the synthesis of dibenzothiophene derivatives usually require harsh conditions and can suffer from low atom economy. Methods based on C–H bond functionalization can offer alternative pathways. However, they generally require expensive metal catalysts or stoichiometric amounts of iodide. Transition-metal- and iodine-free methods for the preparation of dibenzothiophene derivatives would be useful.
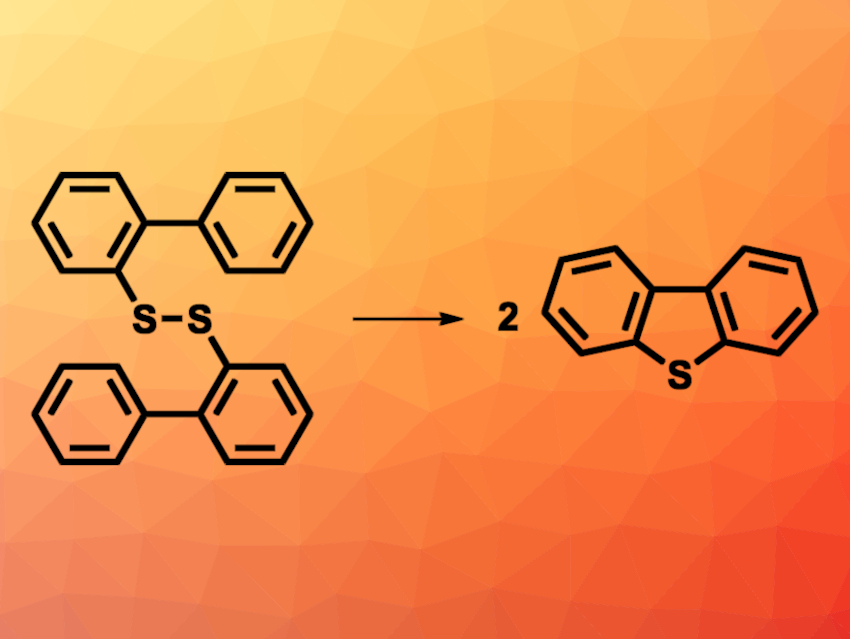
Koichi Mitsudo, Seiji Suga, Okayama University, Japan, and colleagues have developed an electrochemical synthesis of dibenzothiophene derivatives that avoids the need for transition metals or iodine (example reaction pictured). The team started from different bis(biaryl) disulfides and performed an electrochemical cyclization in the presence of Bu4NBr, water, and LiClO4, using CH3NO2 as the solvent. The reactions were performed at 100 °C in an undivided electrochemical cell with two Pt electrodes.
The desired products were obtained in mostly moderate to high yields. Dibenzothiophenes with electron-withdrawing groups such as CF3 at the outer arene ring were obtained in low yields. The team proposes a mechanism in which Br– from Bu4NBr is oxidized to generate a [Br+]-type species. This species reacts with the bis(biaryl) disulfide to give a biphenylsulfanyl bromide (Ar2–S–Br). This is followed by an intramolecular nucleophilic attack of one arene ring at the sulfur atom, which forms the five-membered ring, and deprotonation to give the desired product.
- Electrochemical Synthesis of Dibenzothiophenes via Intramolecular C–S Cyclization with a Halogen Mediator,
Koichi Mitsudo, Yuri Tachibana, Eisuke Sato, Seiji Suga,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c03574