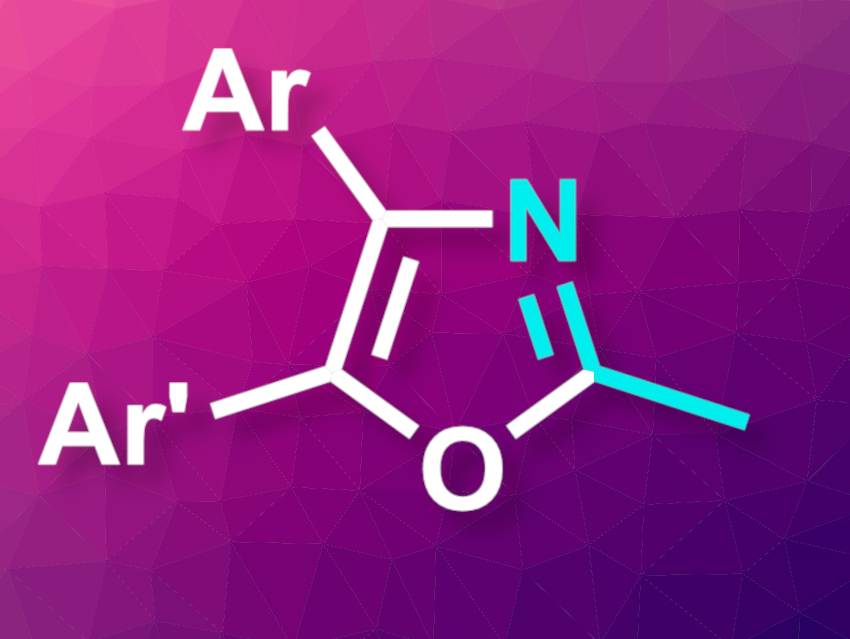
Oxazoles are heterocyclic compounds that are often found in bioactive compounds. Existing methods for their synthesis often suffer from drawbacks such as a need for harsh conditions or a laborious synthesis of the required acyclic precursors. New and improved methods for oxazole synthesis are, thus, useful.
Yunfei Zhang, China Agricultural University, Beijing, and colleagues have developed an electrochemical method for the construction of polysubstituted oxazoles (general structure pictured). The team used easily accessible aryl-substituted ketones as substrates that were reacted with acetonitrile, which serves as both solvent and reactant. The reactions took place in a divided electrochemical cell at room temperature. The researchers used carbon felt as both the anode and the cathode and the transformation was performed in the presence of acid anhydrides, i.e., trifluoroacetic anhydride (TFAA) and acetic anhydride (Ac2O), as activators, triphenylamine derivatives as catalysts, and LiClO4 as the electrolyte.
The desired substituted oxazoles were obtained in generally high yields and the reaction showed a good functional group tolerance. According to the researchers, this green protocol under mild conditions might be useful, e.g., in pesticide, pharmaceuticals, and materials science.
- Electrochemical Synthesis of Polysubstituted Oxazoles from Ketones and Acetonitrile,
Liang Bao, Chen Liu, Wenyi Li, Jiage Yu, Min Wang, Yunfei Zhang,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02252