Formic acid (HCO2H) can be prepared via an electrochemical reduction of CO2 with high efficiency at industrially useful current densities. However, the demand for formic acid is low compared with the amount of CO2 emissions. Thus, to utilize CO2 as sustainably as possible, further processes that use formic acid as a feedstock would be useful.
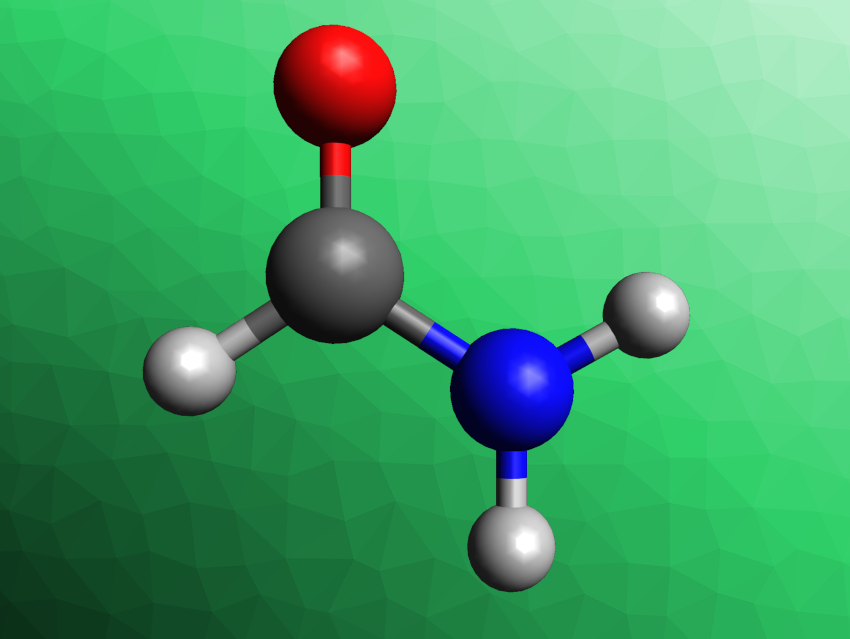
Yifu Yu, Tianjin University and Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, China, Bin Zhang, Tianjin University, and colleagues have developed an electrochemical process for the conversion of formic acid and nitrite into formamide (HCONH2) over a copper catalyst. Formamide is the simplest amide and is usually produced from CO and NH3 in an energy-intensive process that causes high CO2 emissions. There is a much higher demand for formamide than for formic acid.
The team screened different metal electrocatalysts for the synthesis of formamide via the co-reduction of formate and nitrite, i.e., Co, Fe, Ni, Cu, C, Mo, and Pt. They found that a copper foil showed the highest activity and a Faradaic efficiency of 6.9 %. They optimized the catalyst by preparing low-coordinated copper via electrochemical pre-reduction, using Cu2O nanocubes as precursors. Using this catalyst, the researchers achieved an average Faradaic efficiency for the production of formamide of 29.7 %.
As a nitrogen source, the nitrite can also be replaced with nitrate, a common pollutant in water. In addition, the method can also be used to upgrade ethanoic acid (H3CCO2H) to acetamide. According to the team, the approach could provide a sustainable way to construct products with C–N bonds using CO2-derived feedstocks under mild conditions.
- Electrochemical Upgrading of Formic Acid to Formamide via Coupling Nitrite Co-Reduction,
Chengying Guo, Wei Zhou, Xianen Lan, Yuting Wang, Tieliang Li, Shuhe Han, Yifu Yu, Bin Zhang,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c05660