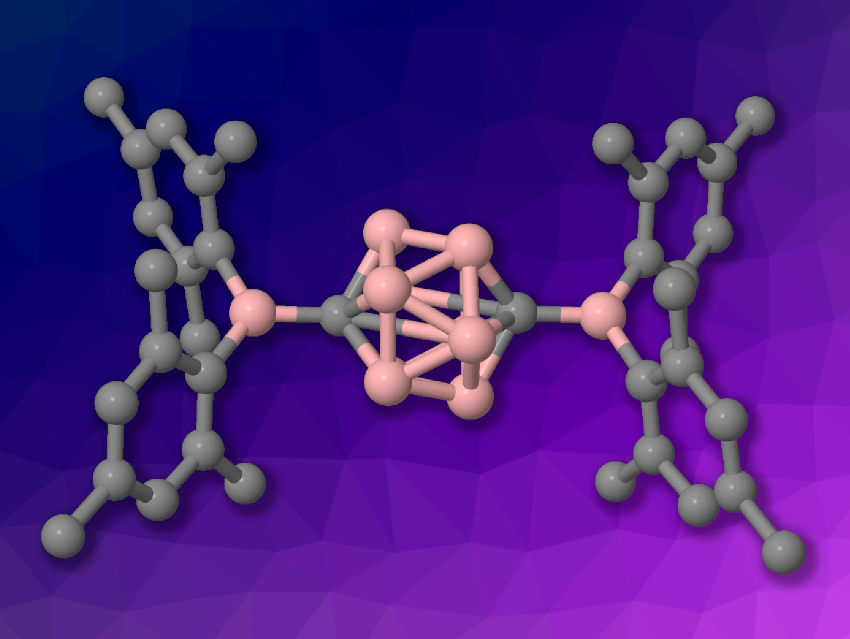
Carboranes are clusters composed of boron, carbon, and hydrogen atoms. They have applications, e.g., in materials chemistry. Often, functional materials in optoelectronics are based on two-dimensional π-conjugated systems. Some carboranes can be considered 3D aromatic systems, with delocalized skeletal electrons, which could be useful for the development of conjugated materials. However, the conjugation between carborane clusters and the attached substituents is usually not very strong.
Christoph Lambert, Holger Braunschweig, University of Würzburg, Germany, Lei Ji, Northwestern Polytechnical University (NPU), Xi’an, China, and colleagues have reduced a carborane with two dimesitylboryl (BMes2) substituents on opposing sides (1,12-bis(BMes2)-p-carborane) and found that the resulting radical anion shows extensive electron delocalization across the carborane “bridge”. The team synthesized four different substituted carboranes, with either one or two BMes2 groups bound to carbon atoms of p– and m-carboranes, respectively. After initial cyclic voltammetry (CV) studies, they chemically reduced the bis–BMes2-substituted p-carborane and m-carborane using [K(18-crown-6)(THF)2] naphthalenide and characterized the resulting products using X-ray diffraction, electron paramagnetic resonance (EPR) spectroscopy, and density functional theory (DFT) calculations.
The researchers found that the radical anion formed by the reduction of 1,12-bis(BMes2)-p-carborane (simplified structure pictured above) features an unpaired electron that is fully delocalized across the p-carborane bridge between the two boron centers of the BMes2 substituents. This allows electronic communication between the boron centers. The team proposes that this strong conjugation is due to the overlap of the carborane’s lowest unoccupied molecular orbital (LUMO) and the LUMO of the boron-based substituents. Overall, the work shows that closo-carboranes can serve as efficient conjugating bridges between suitable substituents.
- Full Electron Delocalization across the Cluster in 1,12-bisBMes2–p-carborane Radical Anion,
Lin Wu, Xinning Zhang, Michael Moos, Ivo Krummenacher, Maximilian Dietz, Arumugam Jayaraman, Rüdiger Bertermann, Qing Ye, Maik Finze, Michael Wenzel, Roland Mitric, Christoph Lambert, Holger Braunschweig, Lei Ji,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c03873