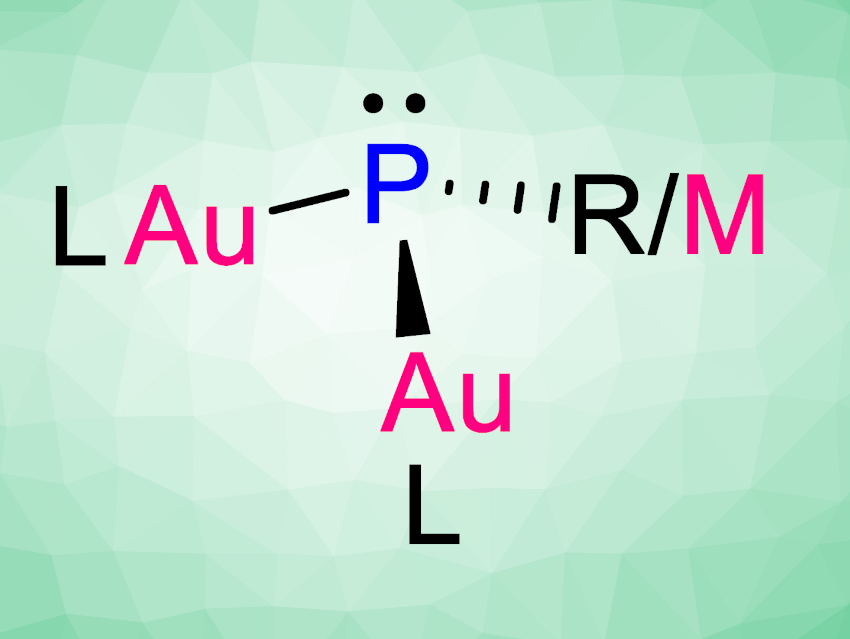Phosphines are commonly used ligands in synthetic chemistry. Their electronic properties and steric bulk can be tuned by changing the substituents at the trivalent phosphorus center. This provides access to numerous phosphines with diverse properties. However, expanding the limits of electron-rich phosphines has been somewhat challenging.
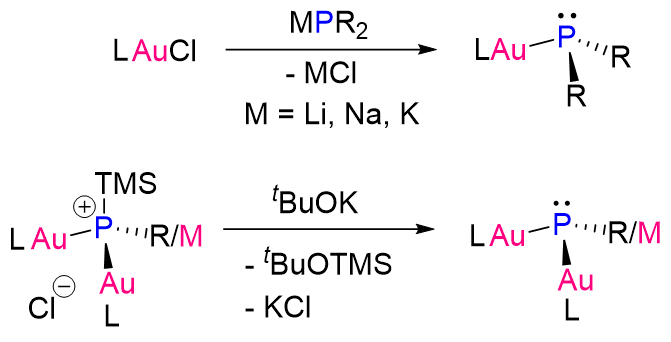
Liu Leo Liu, Southern University of Science and Technology, Shenzhen, China, and colleagues have synthesized a family of mono-, di-, and tri-gold-substituted phosphines (AuPhos, pictured). The compounds are easily prepared via either a salt metathesis reaction or a desilylation reaction (see below, R = e.g., Ph; L = e.g., N-heterocyclic carbenes).
The resulting phosphine compounds can be considered extremely electron-rich phosphorus superbases. This is due to a d–p lone pair π-repulsion between the non-bonding d-electrons at the gold atoms and the lone pair at phosphorus. The electron–rich nature of AuPhos compounds can, thus, be easily tuned by modifying the π–accepting ability of the ligands at the gold centers.
The team found that a series of multinuclear transition-metal complexes were accessible via the coordination of metal precursors with AuPhos ligands. A palladium complex of this type catalyzed the C–N coupling of 4-chlorotoluene and morpholine, and an iridium complex was found to catalyze the decarbonylation of 2-naphthaldehyde.
- Free Metallophosphines: Extremely Electron‐Rich Phosphorus Superbases that are Electronically and Sterically Tunable,
Rui Wei, Shaoying Ju, Liu Leo Liu,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202205618