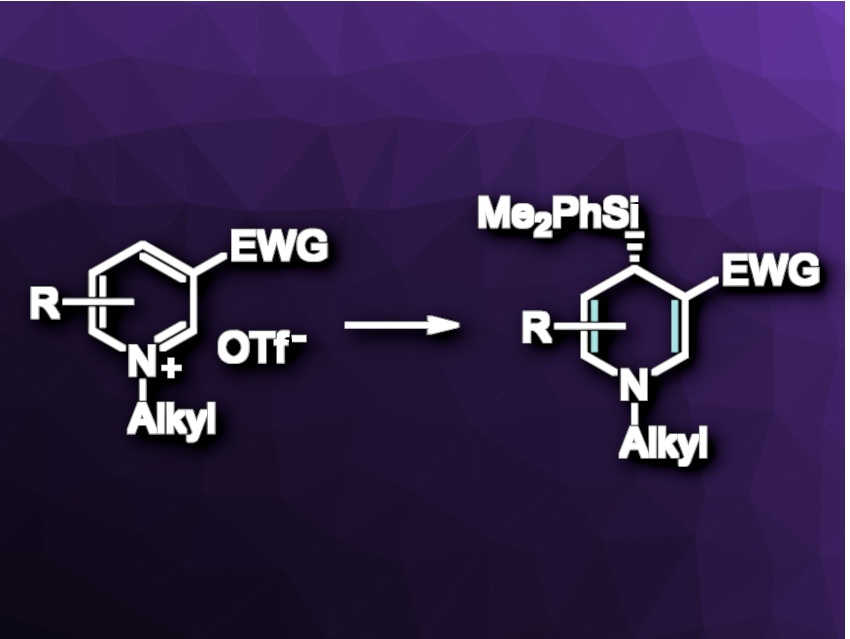
Dearomatization reactions can be used to obtain three-dimensional structures from two-dimensional aromatic substrates with high levels of regio- and enantiocontrol. For pyridine units, for example, the dearomatization leads dihydropyridines, which can be pharmaceutically relevant or further functionalized to give synthetically valuable piperidine building blocks.
Martin Oestreich, Technical University of Berlin, Germany, and colleagues have developed a copper-catalyzed C4-selective addition of silicon nucleophiles to prochiral pyridinium triflates (general reaction pictured). The team used Cu(CH3CN)4PF6 as a precatalyst and (R,R)-Ph-BPE (1,2-bis[(2R,5R)-2,5-diphenylphospholan-1-yl]ethane) as a chiral ligand, LiOtBu as a base, Me2PhSi–Bpin as a precursor for the nucleophile, and a mixture of 1,4-dioxane and water as the solvent. The reactions were performed at room temperature.
Under these conditions, the dearomatization proceeds with mostly excellent enantioselectivities and moderate to excellent yields. The researchers found that the approach requires a carbonyl group at C3, likely to preorganize and direct the nucleophilic attack towards C4. The prepared 4-silylated 1,4-dihydropyridines can be further functionalized to give functionalized piperidine derivatives.
- Enantioselective Dearomatization of Pyridinium Salts by Copper‐Catalyzed C4‐Selective Addition of Silicon Nucleophiles,
Yao Xiao, Zhi-Yuan Zhao, Sebastian Kemper, Elisabeth Irran, Martin Oestreich,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202407056