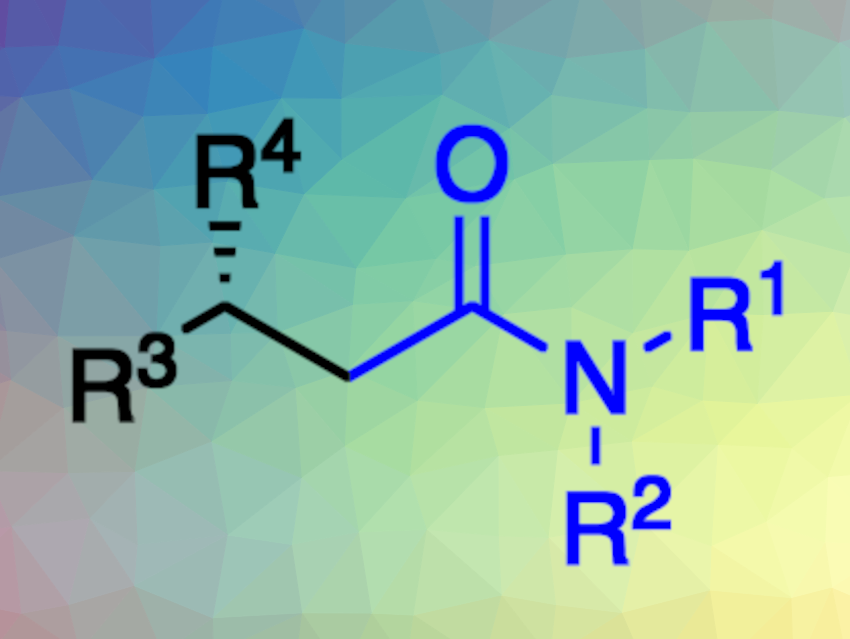
Chiral amides are commonly found, e.g., in pharmaceuticals and natural products. The enantioselective hydroaminocarbonylation of olefins is a straightforward method for preparing enantioenriched amides. However, this approach requires toxic CO gas, often at high pressures.
Stephen L. Buchwald and colleagues, Massachusetts Institute of Technology (MIT), Cambridge, USA, have developed an asymmetric hydrocarbamoylation of alkenes that uses readily available carbamoyl chlorides as practical carbamoylating reagents (two variants of the reaction pictured below). The team used dual copper hydride and palladium catalysis to enable the transformation.
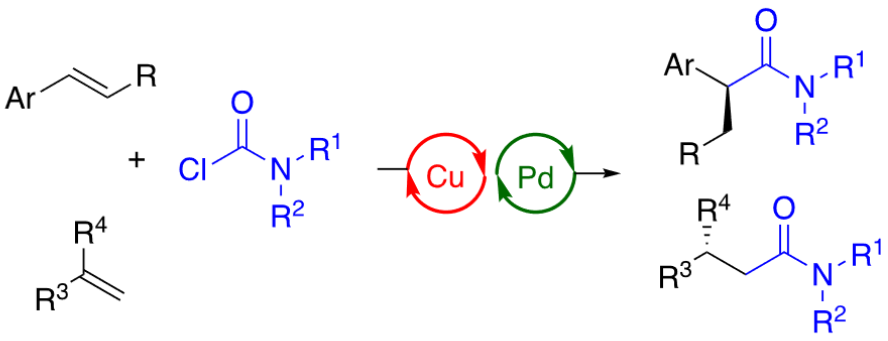
They used Cu(OAc)2 or CuOAc as copper catalysts together with (R)-DTBM-SEGPHOS as a chiral phosphine ligand, [Pd(cinnamyl)Cl]2 or G3-dimer (Buchwald precatalyst) as palladium catalysts together with SPhos (2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl) or BrettPhos (2-dicyclohexylphosphino-3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl) as biarylphosphine ligands, sodium pivalate or potassium benzoate as a base, (MeO)2MeSiH as a hydride source, and tetrahydrofuran (THF) as the solvent. The reactions were performed at 40 °C.
Different types of alkenes, including vinyl arenes, terminal olefins, and 1,1-disubstituted alkenes, participate in the reaction. The team showed that both α- and β-chiral amides could be prepared in good yields and excellent enantioselectivity using this approach. Additionally, the transformation was able to accommodate a broad range of functional groups and heterocycles.
- Enantioselective Hydrocarbamoylation of Alkenes,
Sheng Feng, Yuyang Dong, Stephen L. Buchwald,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202206692