Nucleophilic vinylic substitution (SNV) at sp² carbons allows direct C–C and C–heteroatom bond formation, making it valuable for synthesizing heterocycles and multifunctional alkenes. While halogen-based SNV reactions show good enantioselectivity, nitro groups present challenges due to poor leaving ability and tendency for side reactions.
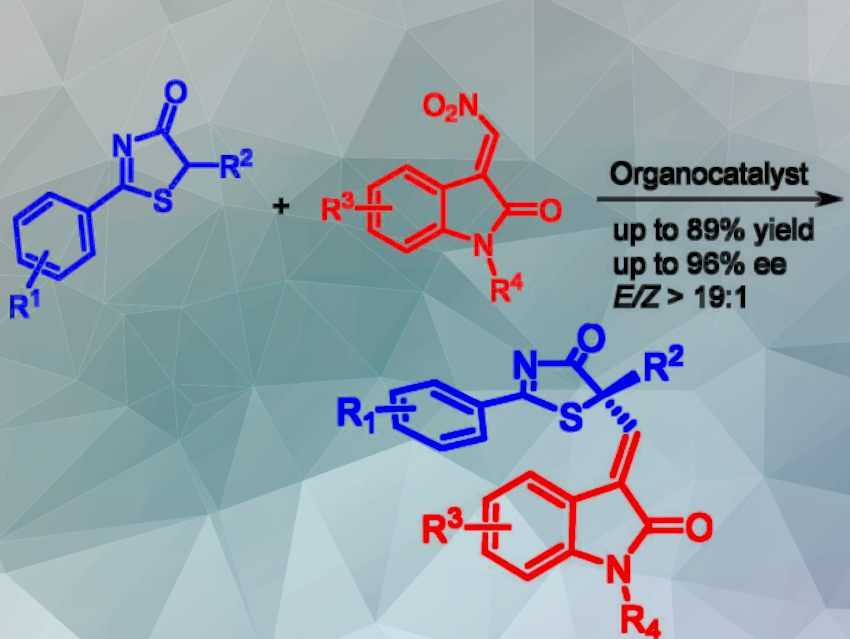
Weiwu Ren and colleagues, Ocean University of China, Qingdao, and Qingdao Marine Science and Technology Center, have developed an asymmetric catalytic SNV reaction of 5H-thiazol-4-ones and nitroolefins for the synthesis of 3-alkenyl oxindoles (pictured).
The enantioselective SNV reaction with a nitro group as the leaving group proceeded smoothly to afford 3-alkenyl oxindoles in good yields with high regio- and enantioselectivity. The reaction showed broad substrate applicability. A quaternary carbon stereocenter, which was substituted by sp² carbon, was constructed under mild reaction conditions.
- Enantioselective Nucleophilic Vinylic Substitution of Nitroolefins for the Synthesis of 3-Alkenyl Oxindoles,
Yuan Pan, Zhou Pan, Kangkang Qi, Zhenyu Shi, Weiwu Ren,
J. Org. Chem. 2025.
https://doi.org/10.1021/acs.joc.5c00190