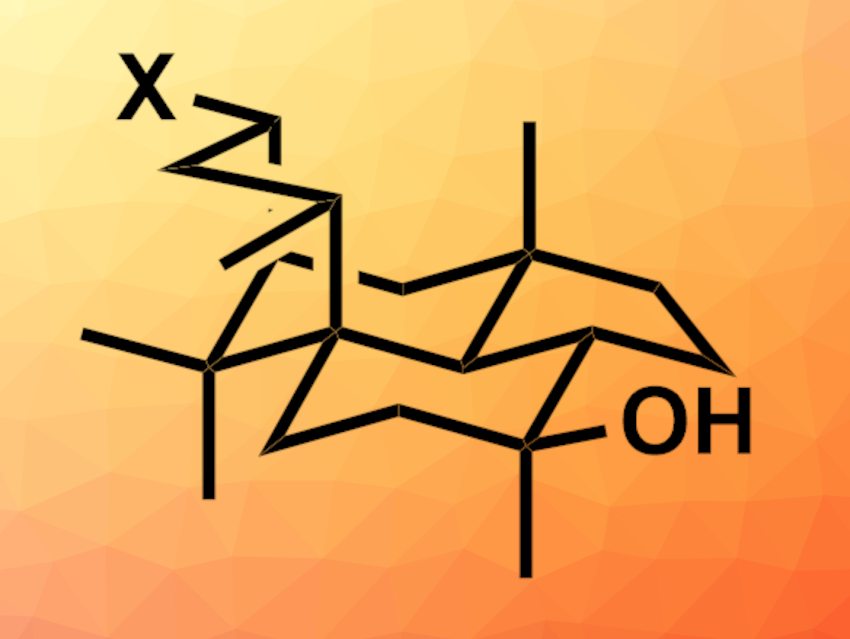
Wickerol A (pictured, X = H) is a tetracyclic compound that was isolated from a fungus. The compound has shown potent antiinfluenza activity. Wickerol B only differs from wickerol A by an OH group (pictured, X = OH). Only small amounts of these substances have been isolated from natural sources. Thus, the development of synthesis routes that give wickerols A and B would be useful for further studies of their biological activities. With the sterically congested bridging six-membered ring and three quaternary carbons, the compounds are challenging to synthesize.
Christopher D. Vanderwal, University of California, Irvine, USA, and colleagues have performed enantioselective syntheses of wickerols A and B. The team started from cyclohexenone and used a series of conjugate addition reactions to build the fused tricyclic core of the compounds. They employed a Claisen rearrangement to introduce the C6 methyl substituent, which was otherwise difficult to install. To close the final, strained, bridging ring, the researchers used a Prins cyclization.
The team completed the synthesis of wickerol B in 23 steps from cyclohexenone. Removal of the hydroxy group at C8 via a Barton–McCombie deoxygenation then led to wickerol A. According to the researchers, the insights gained in the development of this synthesis could be useful to synthetic organic chemists.
- Enantioselective Syntheses of Wickerols A and B,
Jonathan Chung, Joseph S. Capani, Matthias Göhl, Philipp C. Roosen, Christopher D. Vanderwal,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c00448