The functionalization of compounds with fluorine atoms can be useful for tuning their physicochemical properties, in particular, in medicinal chemistry. Incorporating fluorinated amino acids into peptides is interesting in this context. CF2H groups, for example, can act as hydrogen-bond donors and change the lipophilicity of compounds. However, amino acids with such substituents are not well-studied. For β,β-difluoroalanine (DfAla), for example, there are limited examples of reported enantioselective syntheses, and this non-natural amino acid had not been incorporated into peptides in an enantiopure form so far.
Benoît Crousse, Université Paris-Saclay, CNRS, Orsay, France, Thierry Brigaud, Université Paris-Saclay and CY Cergy Paris Université, CNRS, Cergy-Pontoise, France, and colleagues have developed a straightforward synthesis of (R)- and (S)-difluoroalanine, incorporated the amino acid into diastereomerically pure tripeptides, and evaluated how they affect the hydrophobicity of the peptides. The team started with the condensation of difluoroacetaldehyde ethylhemiacetal with (R)-phenethylamine to obtain an imine. A Strecker-type reaction with trimethylsilyl cyanide (TMSCN) then gave an amino nitrile as a mixture of diastereomers.
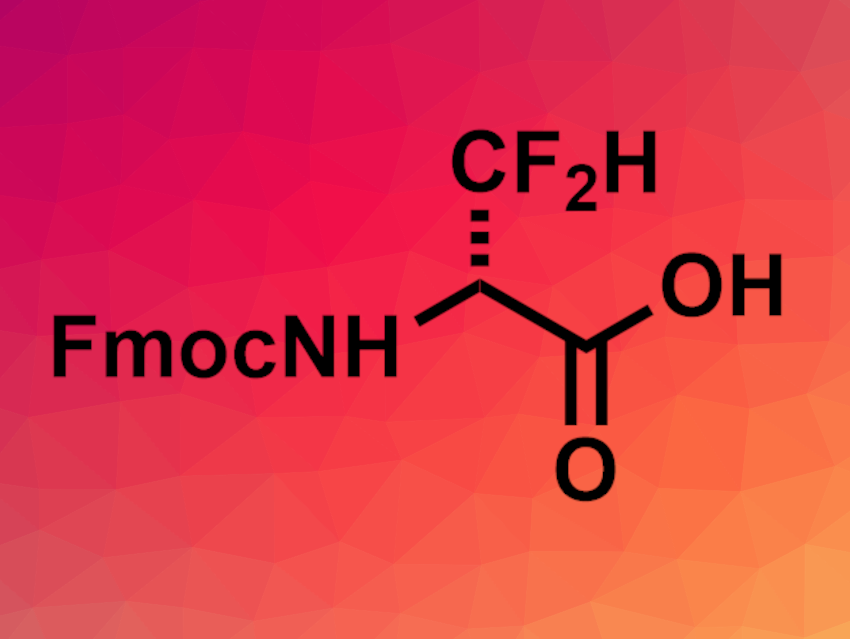
The diastereomers were separated by column chromatography. Hydrolysis of the nitrile function, debenzylation, and fluorenylmethoxycarbonyl protecting group (Fmoc)-protection of the amino group then gave enantioenriched Fmoc-amino amides. Acidic hydrolysis of the amide group then gave Fmoc-difluoroalanine (pictured).
The synthesized N-protected fluorinated amino acid was then used in peptide coupling reactions to form tripeptides. The team determined the local hydrophobicity change caused by the CF2H group by measuring the chromatographic hydrophobicity index of the tripeptide H2N-Ala-DfAla-Leu-OH and comparing it with those of suitable reference tripeptides. The team found that the CF2H group provides nearly the same hydrophobic contribution as the side chain of isoleucine, but the CF2H group has a smaller van der Waals volume. This could be useful in fine-tuning peptide properties.
- Difluoroalanine: Synthesis, Incorporation in Peptides, and Hydrophobic Contribution Assessment,
Yazid Boutahri, Khoubaib Ben Haj Salah, Nicolas Tisserand, Nathalie Lensen, Benoît Crousse, Thierry Brigaud,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02853