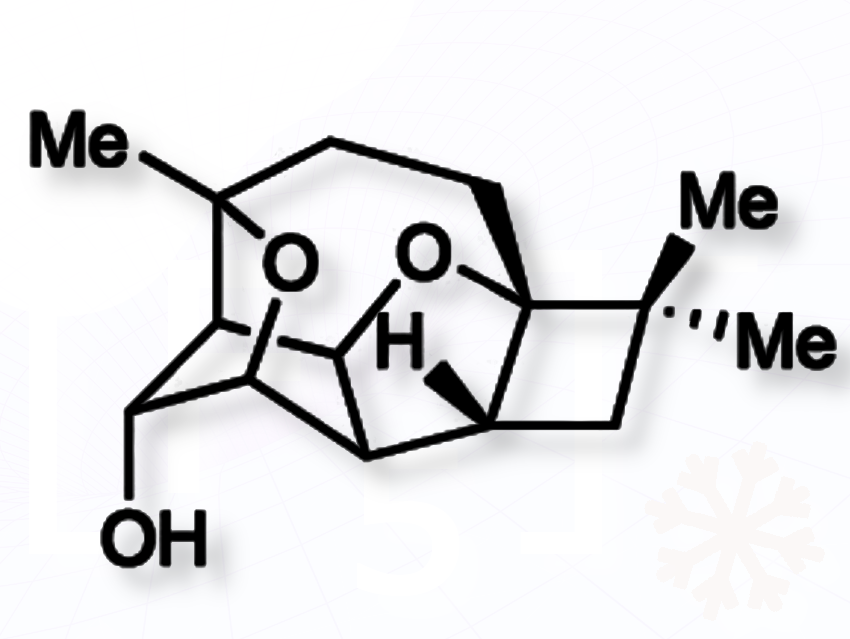
Jieping Zhu and colleagues, Ecole Polytechnique Fédérale de Lausanne, EPFL, Switzerland, have accomplished the first enantioselective total synthesis of the natural product (+)-punctaporonin U in only six steps. This cage-like pentacyclic sesquiterpene bearing eight contiguous stereocenters was originally isolated from the Chaetomium globosum fungi.
The team started their synthesis from the enantioenriched building block 4-[(tert-butyldimethylsilyl)oxy]cyclopent-2-en-1-ones. The synthesis features a sequence of distinctive transformations, including a rare cis-selective Mukaiyama–Michael addition, a domino oxa-Michael/aldol/bromination process forging the fused five-membered and 1,4-bridged seven-membered rings, and an intramolecular SN2 displacement to close a final oxygen bridge establishing the 1,3-bridged tetrahydrofuran moiety.
This synthesis provides a practical way of accessing a family of biologically active sesquiterpenes. It enables studies of their antibacterial and anticancer potential, and illustrates how multi-step cascade reactions can efficiently build highly strained molecular frameworks, according to the researchers.
- Concise Enantioselective Total Synthesis of (+)-Punctaporonin U
Paul Gri, Qian Wang, Jieping Zhu
J. Am. Chem. Soc. 2025.
https://doi.org/10.1021/jacs.5c15423