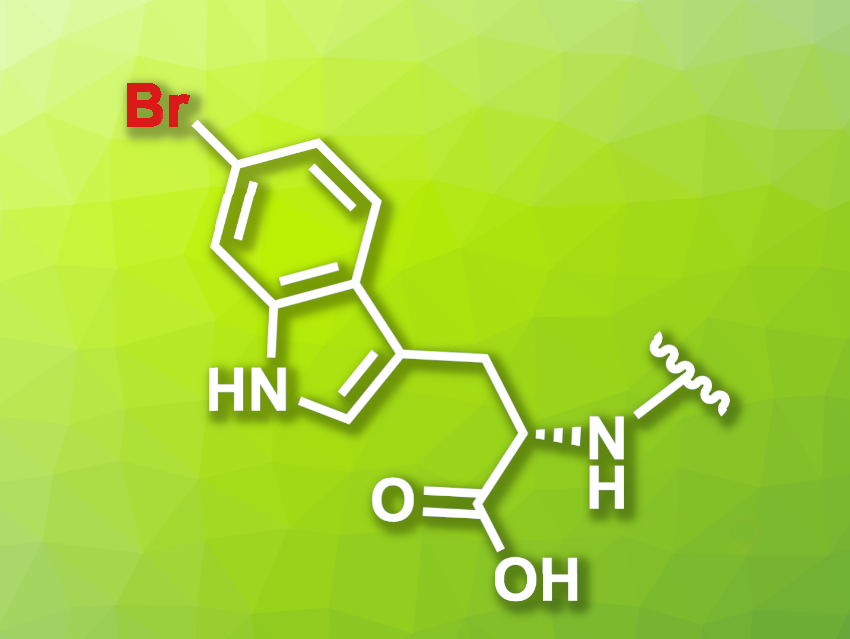
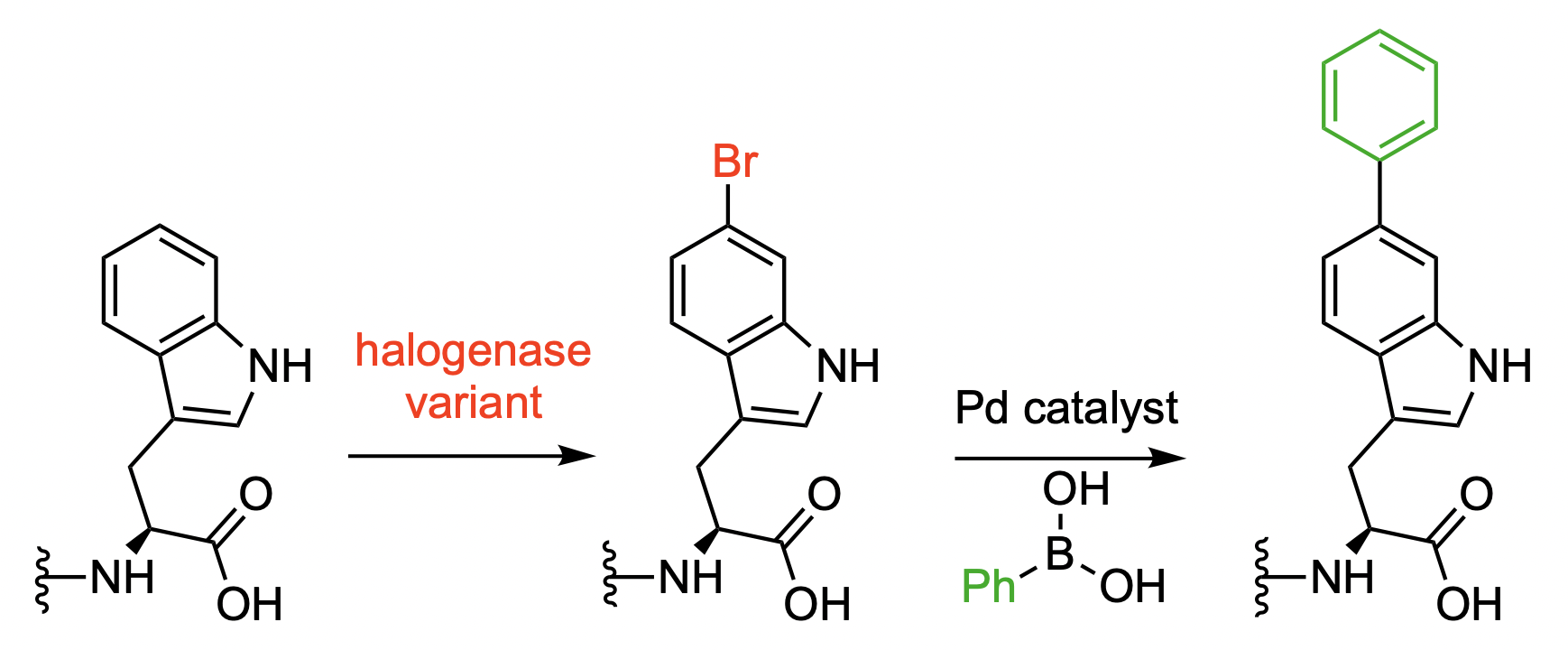
Reactions that functionalize proteins can allow researchers to observe their behavior, e.g., by attaching fluorophores to them. One way to achieve this type of modification is the enzymatic bromination of a protein, followed by transition-metal-catalyzed transformations such as cross-coupling reactions.
Norbert Sewald, Bielefeld University, Germany, and colleagues have developed a modified halogenase that can be used for the bromination of proteins, which are tagged using a tetrapeptide with a C-terminal tryptophane unit. The team modified the native tryptophan (Trp) 6-halogenase Thal using a semi-rational protein engineering approach. The resulting mutant Thal-AAC, which was modified in three positions, shows a preference for bromination over chlorination and good regioselectivity for the C6 position.
To find an optimal peptide tag, the researchers screened peptide libraries. They found that the amino acid sequence YNIW (tyrosine-asparagine-isoleucine-tryptophan), a tag they call BromoTrp, is optimal for the transformation.
The researchers obtained preparative amounts of brominated protein in one cultivation and purification step using E. coli cultures that produce both the engineered enzyme and the tagged proteins. A Pd-catalyzed Suzuki–Miyaura cross-coupling of the resulting aryl bromide groups was then used to create biaryl units (pictured below). This serves as a proof-of-concept showing that further functionalizations are possible.
- Enzymatic Peptide and Protein Bromination: The BromoTrp Tag,
Nicolai Montua, Paula Thye, Pia Hartwig, Mathias Kühle, Norbert Sewald,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202314961