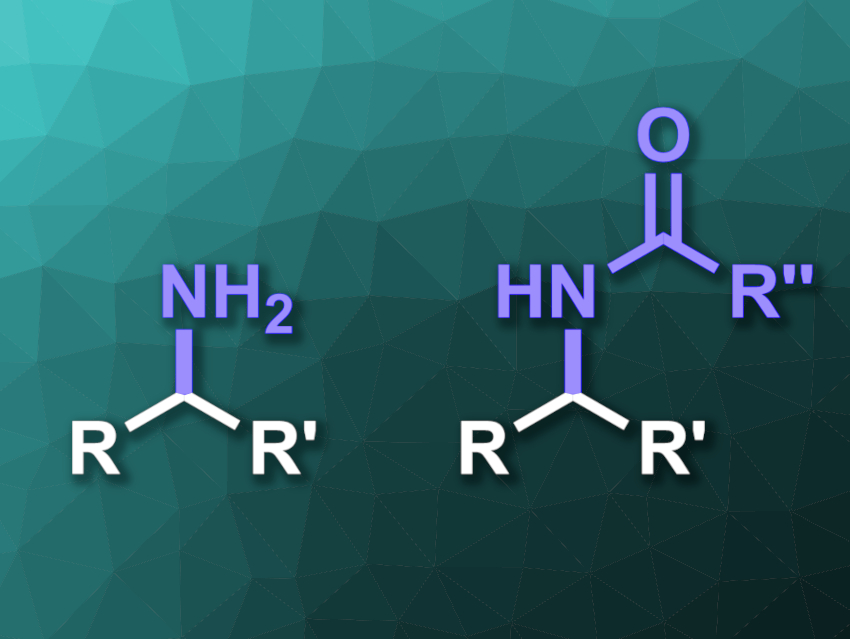
Enzymes can catalyze C–H functionalizations in biological systems. This could also be useful in organic synthesis. However, enzymatic C–H functionalization with heteroatoms is generally restricted to oxygen and halogen atoms. The analogous direct enzymatic insertion of nitrogen into unactivated C–H bonds is unknown in Nature.
Soumitra V. Athavale, Frances H. Arnold, California Institute of Technology, Pasadena, USA, Jennifer S. Hirschi, Binghamton University, NY, USA, and colleagues have used the directed evolution of enzymes to find enzymes that can selectively aminate and amidate unactivated C(sp3)–H bonds (general product structures pictured). The team used heme-containing nitrene transferases as starting points. They performed iterative rounds of 1) mutation to change the enzymes followed by 2) testing the resulting variants for their activity in the desired reactions. Variants with improved activity were then used as a new starting point for the next round of directed evolution.
The optimized enzymes the team obtained by this approach were used for the amination or amidation of methyl- and ethylcyclohexane as model substrates. The researchers found that the evolved enzymes show high site- and stereoselectivity and have a broad substrate scope. According to the team, further directed evolution might lead to enzymes that are selective for other sites. Overall, the developed enzymes provide a new route for the synthesis of nitrogen-containing molecules.
- Enzymatic Nitrogen Insertion into Unactivated C–H Bonds,
Soumitra V. Athavale, Shilong Gao, Anuvab Das, Sharath Chandra Mallojjala, Edwin Alfonzo, Yueming Long, Jennifer S. Hirschi, Frances H. Arnold,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08285