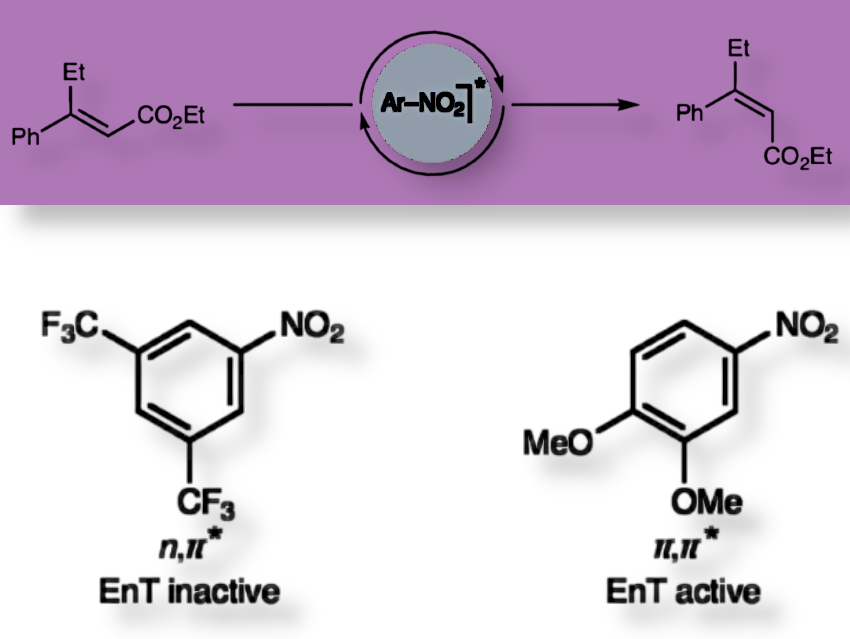
Enrique M. Arpa, Daniele Leonori, and colleagues, RWTH Aachen University, Germany, have explored how simple nitroarene molecules can act as light-driven catalysts, transferring energy to other molecules without needing high-energy UV light.
Nitroarenes with excited states centered on the aromatic ring (3π,π*) work much better as catalysts than those centered on the nitro group (3n,π*), showing that where the energy sits in the molecule matters more than the usual energy-matching rules. The 3π,π* states have longer lifetimes and better overlap with the substrate’s orbitals, making energy transfer more efficient. In contrast, 3n,π* states are less reactive because the excitation is localized on the nitro group, which doesn’t interact as well with the substrate.
By using nitroarenes with 3π,π* triplet states, reactions like E-to-Z alkene isomerization or [2+2] cycloadditions happen faster and with visible light, avoiding harsh UV irradiation or expensive metal catalysts.
The team says that this provides a cheap, sustainable alternative to precious-metal catalysts for light-driven chemical reactions, making processes like alkene isomerization and cycloadditions more practical.
- Excited-state configuration controls the ability of nitroarenes to act as energy transfer catalysts
Martin Rihtaršič, Byeongseok Kweon, Piotr T. Błyszczyk, Alessandro Ruffoni, Enrique M. Arpa, Daniele Leonori
Nature Catalysis 2025, 8, 1361–1369.
https://doi.org/10.1038/s41929-025-01453-z