Aluminyl anions are interesting research targets in organometallic chemistry. They are reactive species that are isoelectronic to carbenes, and could provide access to otherwise difficult-to-prepare aluminium-containing compounds. Suitable ligands are needed to stabilize these aluminyl anions. So far, all ligands used to isolate “free” aluminyl anions are chelating ligands, leading to cyclic species.
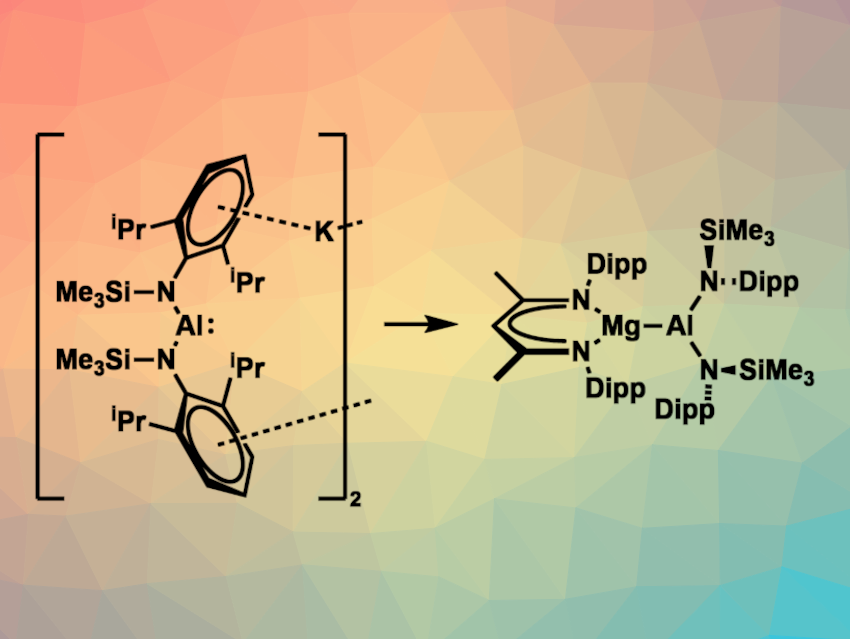
Jamie Hicks, Australian National University, Acton, Australia, David J. Liptrot, University of Bath, UK, and colleagues have synthesized the first acyclic aluminyl anion, stabilized by two bulky monodentate amido ligands. The team first prepared the acyclic bisamido aluminium iodide complex [AlI{N(Dipp)SiMe3}2] from HN(Dipp)SiMe3, lithium aluminium hydride, and trimethylsilyl iodide. The addition of an excess of KC8 to [AlI{N(Dipp)SiMe3}2] then gave a dimer of an acyclic aluminyl anion, bridged by potassium ions—i.e., K2[Al{N(Dipp)SiMe3}2]2 (pictured above on the left). According to the researchers, this is the first structurally characterized acyclic aluminyl anion.
The compound can act as a nucleophilic source of aluminium, which the team showed by reacting it with electrophilic [(Dippnacnac)MgI(OEt2)] (Dippnacnac = [{(Dipp)NC(CH3)}2CH]). They obtained [(Dippnacnac)MgAl{N(Dipp)SiMe3}2] (pictured above on the right), which can be considered a magnesium complex of a monomeric acyclic aluminyl anion.
- An acyclic aluminyl anion,
Ross A. Jackson, Aidan J. R. Matthews, Petra Vasko, Mary F. Mahon, Jamie Hicks, David J. Liptrot,
Chem. Commun. 2023.
https://doi.org/10.1039/D3CC01317K