Since their discovery in 2005, cyclic (alkyl)(amino)carbenes (CAACs) have gained increasing attention, and their use as starting materials and co-substituents is a topical field of interest. These carbenes are more nucleophilic and electrophilic than their NHC counterparts. However, unlike to white phosphorus (P4), the reactivity of yellow arsenic (As4) towards carbenes remained unexplored, although the resulting products bear high potential as ligands or in materials sciences. This is due to the toxicity, light- and air-sensitivity of yellow arsenic, and the impossibility to carry
out stoichiometric reactions due to its autocatalytic conversion to grey arsenic.
Manfred Scheer, University of Regensburg, Germany, and colleagues have reacted different CAACs with As4. Compared to the lighter P4, studies on the reactivity of As4 pose the serious challenge of performing all synthetic steps under strict exclusion of light. The reaction leads to aggregation, fragmentation, and rearrangement of As4. The resulting products were isolated and characterized using spectroscopic and crystallographic methods.
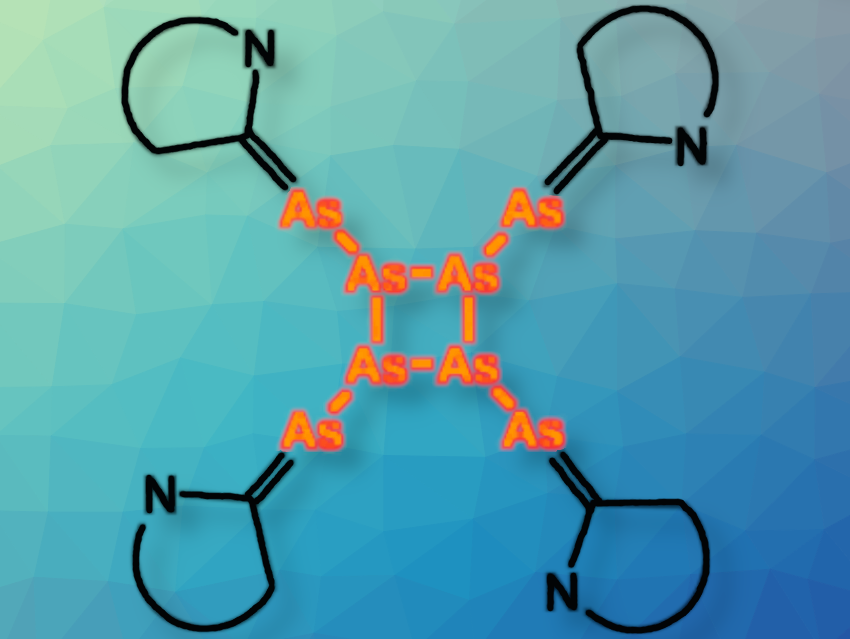
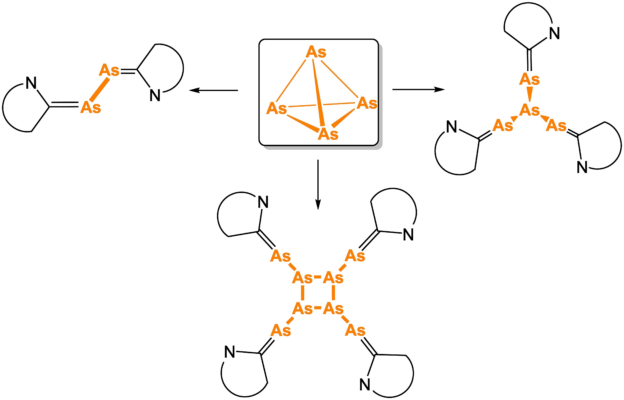
The products included [(CAAC-n)2(μ,η1:1-As2)] (n = 1, 4) (pictured below on the left), [(CAAC-2)3(μ3,η1:1:1-As4)] (pictured below, right), and [(CAAC-3)4(μ4,η1:1:1:1– As8)] (pictured below, bottom). They contain As2, As4, or As8 units and represent the first examples of CAACs-substituted products of yellow arsenic. The reaction outcome depends on the sterics of the respective CAAC. These products are less stable than their phosphorus analogues, which also affects the isolated yields. Only the thermodynamically most stable products could be isolated.
The team also compared the reactivity of As4 with that of P4 and the interpnictogen compound AsP3. This lead to the formation of a series of phosphorus-containing derivatives such as [(CAAC-3)3(μ3,η1:1:1-P4)], [(CAAC-3)4(μ4,η1:1:1:1-P8)], and [(CAAC-3)3(μ3,η1:1:1-AsP3)]. To better understand the formation pathway of these products, the team performed DFT computations.
- Reactivity of Yellow Arsenic towards Cyclic (Alkyl)(Amino) Carbenes (CAACs),
Maria Haimerl, Christoph Schwarzmaier, Christoph Riesinger, Alexey Timoshkin, Mohand Melaimi, Guy Bertrand, Manfred Scheer,
Chemistry – A European Journal 2023.
https://doi.org/10.1002/chem.202300280