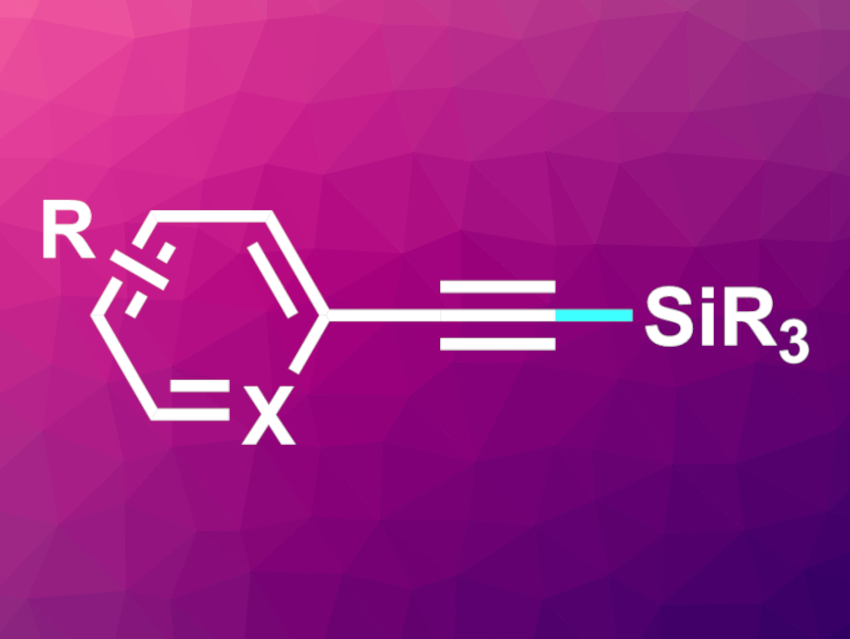
Alkynylsilanes (example pictured) are useful intermediates in organic synthesis. They can be of interest, e.g., in medicinal or materials chemistry. The direct activation of alkynes and silanes to form C(sp)–Si bonds could provide a straightforward path to alkynylsilanes. Photocatalysis might be a promising approach to these reactions, but so far, there had been no examples of photochemical C(sp)–Si bond formation reactions that give alkynylsilanes.
Li-Zhu Wu, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, and University of the Chinese Academy of Sciences, both Beijing, and colleagues have developed the first example of a C(sp)–H/Si–H cross-coupling reaction by photocatalysis (general product structure pictured). The team used a variety of benzene alkynes and aromatic heterocyclic terminal alkynes as substrates and reacted them with different silanes, using CuCl as a catalyst, Ph(CO)O2tBu as an oxidant, and a mixture of acetonitrile and methanol as the solvent. The reactions were performed under blue LED light and an inert atmosphere at room temperature.
The desired alkynylsilanes were obtained in moderate to excellent yields. The approach was also successfully used in a scaled-up reaction (2 mmol) and in a continuous-flow system. The team proposes a reaction mechanism that involves the formation of a Cu(I)-phenylacetylide under visible-light irradiation, followed by a single-electron transfer to the peroxide to give a Cu(II)-phenylacetylide. The resulting alkoxy radicals activate the Si–H bond, leading to silicon radicals that react with the Cu(II)-phenylacetylide and then give the desired product.
- Direct C(sp)–H/Si–H Cross-Coupling via Copper Salts Photocatalysis,
Qi-Chao Gan, Zi-Qi Song, Chen-Ho Tung, Li-Zhu Wu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02022