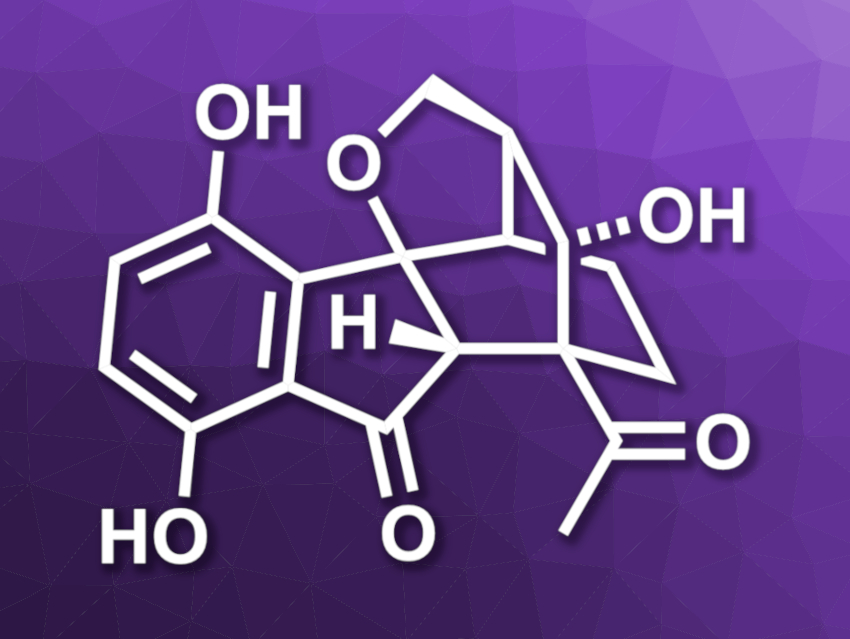
Lucidumone (pictured) is a natural product and was isolated from mushrooms that are commonly used in traditional medicine in China and Japan. The compound has shown interesting bioactivity. It has a complex structure with a 6/5/6/6/5 pentacyclic core, featuring six contiguous stereocenters. This structure makes it a challenging target for organic synthesis.
Aurélien de la Torre, Université Paris-Saclay, CNRS, Orsay, France, and colleagues have performed the first enantioselective total synthesis of (+)-lucidumone on a gram scale. The team first prepared an indandione fragment from 3,6-dihydroxyphthalonitrile via an anhydride intermediate. A second, cyclohexadiene-based fragment was synthesized from a pyrone and a benzyl enol ether. The two fragments were coupled via a challenging C–O bond formation. A one-pot retro-[4 + 2]/[4 + 2] cycloaddition cascade was then used to form the pentacyclic core. Further functionalization and deprotection steps gave the desired product.
Overall, (+)-lucidumone was obtained in 13 steps (longest linear sequence) and 25.8 % overall yield. The synthesis is scalable, and the team prepared 1.6 g of lucidumone in one batch. This amount is sufficient for further biological studies.
- Gram-Scale Enantioselective Synthesis of (+)-Lucidumone,
Guanghao Huang, Cyrille Kouklovsky, Aurélien de la Torre,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08760