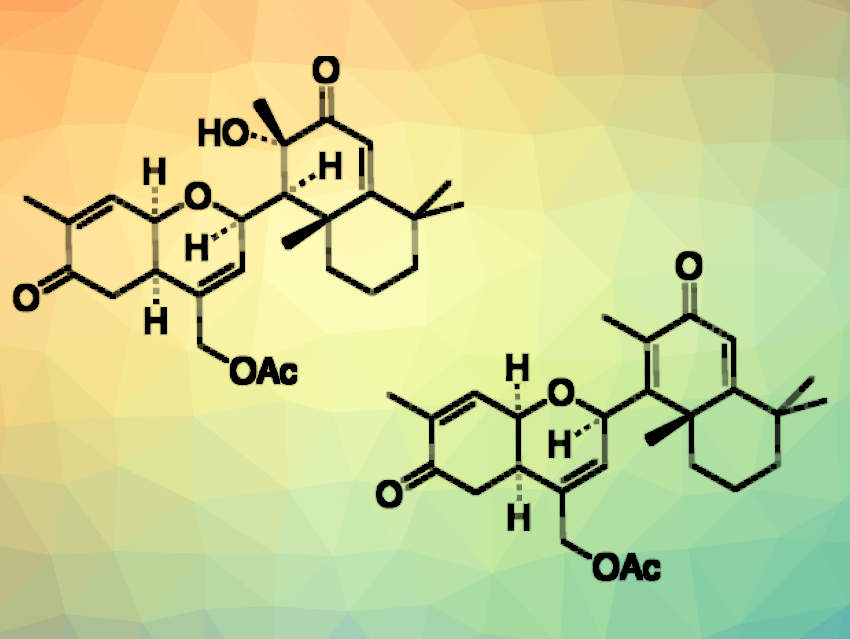
Natural products such as ansellone G (pictured above on the left) or phorbadione (pictured above on the right) belong to a class of marine sesterterpenoids isolated from marine organisms in the Pacific Rim. These compounds combine a hydrobenzopyran unit and different oxidized decalin groups. Some members of this family of compounds have shown interesting bioactivities. Methods for the synthesis of these molecules could, thus, enable further biological investigations.
Mitsuhiro Arisawa, Kenichi Murai, Osaka University, Japan, and colleagues have performed the first total synthesis of ansellone G, which can also be further transformed to give phorbadione. The team started from (+)-sclareolide, which was converted to an aldehyde that serves as a precursor for the decalin unit. This aldehyde was reacted with a chloro-substituted homoallyl alcohol in a key Prins cyclization reaction to create the hydrobenzopyran unit.
Ansellone G was then synthesized by converting the chloro group of the cyclization product to an acetoxy group, using tetrabutylammonium acetate (TBAA). The desired natural product was obtained in 13 % overall yield in ten steps (longest linear sequence).
The team then used Burgess reagent to dehydrate the tertiary alcohol in ansellone G and obtain phorbadione. This product was obtained in an overall yield of 7.2 % and eleven steps (longest linear sequence) from (+)-sclareolide. According to the researchers, the developed strategy could also be useful for the synthesis of other ansellone analogs for biological studies.
- Total Synthesis of Ansellone G and Phorbadione,
Mizushi Yanagihara, Kanae Nakahara, Naoki Kishimoto, Towa Abe, Satoshi Miura, Shogo Misumi, Makoto Sako, Mitsuhiro Arisawa, Kenichi Murai,
J. Org. Chem. 2022.
https://doi.org/10.1021/acs.joc.2c02278