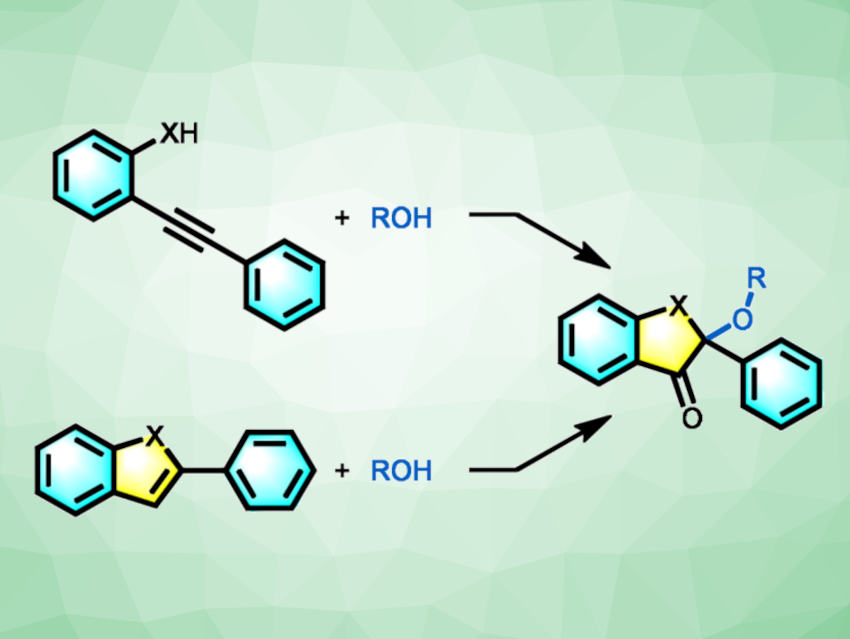
Benzofuranones are heterocycles that are often found, e.g., in natural products, biologically active compounds, or dyes. 2,2-Disubstituted benzofuranone cores. for example, are present in various drugs, such as griseofulvin, maesopsin, and sulfuretin. However, existing methods for the synthesis of 2,2-disubstituted benzofuranones are often hampered by a tedious synthesis of the starting materials, low chemoselectivity, and/or harsh conditions.
Zhonghua Xia, Beijing Institute of Technology, China, and colleagues have developed two flexible methods for synthesizing benzofuran-3(2H)-ones (pictured). One protocol is a gold-catalyzed cycloisomerization of o-alkynyl phenols with alcohols or acids (pictured above in the top row). The team used Ph3PAuCl as the catalyst, Selectfluor as an oxidant, trifluoromethanesulfonic acid (TfOH) as an additive, and MeCN as the solvent. The reactions were performed at 70 °C and the products were obtained in moderate to good yields.
The team also found that a second option is a metal-free treatment of benzofurans with alcohols, acids, or water (pictured above in the bottom row). The team left out the gold catalyst and swapped the o-alkynyl phenol substrates for benzofurans in this approach. In most cases, this approach gave the corresponding benzofuranones in higher yields than the gold-catalyzed cycloaddition reaction. Overall, the two methods provide flexible options for the synthesis of benzofuran-3(2H)-ones from easily available starting materials and with good chemoselectivity and a broad substrate scope.
- Flexible Synthesis of Benzofuranones from o‐Alkynyl Phenols or Benzofurans,
Wenqian Du, Rongjie Yang, Jiawen Wu, Zhonghua Xia,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202201497