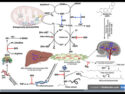
Mark J. Guiltinan and colleagues, Penn State University, University Park, PA, USA, have used the CRISPR–Cas9 gene-editing tool to switch off a cacao gene (TcNPR3) that normally dampens the plant’s natural disease defenses, and then bred the edited plants to remove all foreign DNA.
- CRISPR–Cas9 editing: The teham used CRISPR–Cas9 to introduce mutations in TcNPR3 in T0 (first-generation) cacao plants.
- This involved guide RNAs to direct Cas9 to the TcNPR3 sequence, creating targeted DNA breaks.
- The plant’s repair machinery produced mutations that disrupted TcNPR3 function.
- Initial testing: The T0 plants were assessed in leaf assays for reduced susceptibility to Phytophthora.
- Breeding to remove transgene: T0 plants were crossed with non-transgenic cacao to segregate out the CRISPR–Cas9 DNA (T-DNA), generating progeny that retained the TcNPR3 mutation but lacked foreign DNA.
- Validation: Whole-genome sequencing confirmed single T-DNA insertion sites in T0 plants and identified progeny with only the desired TcNPR3 edits.
The team found that Theobroma cacao L. plants carrying the TcNPR3 mutation showed stronger defense signals and about 42% smaller disease lesions when exposed to the black pod pathogen Phytophthora, a major threat to the global chocolate industry. This strategy creates cacao trees that resist a crop-killing disease without being classified as transgenic, offering a faster, more acceptable path to sustainable chocolate production.
- Reduced Susceptibility to Phytophthora in Non-Transgenic Cacao Progeny Through CRISPR–Cas9 Mediated TcNPR3 Mutagenesis
Mark J. Guiltinan, Lena Landherr, Siela N. Maximova, Dante DelVecchio, Aswathy Sebastian, Istvan Albert
Plant Biotechnology Journal 2025
https://doi.org/10.1111/pbi.70365