Carbazoles are useful compounds due to properties such as their biological activities and their potential as building blocks in materials science. The development of methods for their direct preparation with high atom economy and little waste is, thus, an interesting research target. Among the existing methodologies for the synthesis of carbazoles, the benzoannulation of C-3 functionalized indole derivatives is one of the most useful. Gold catalysis has become a powerful tool for the preparation of high-value chemicals from easily available starting materials due to its ability to promote intramolecular cyclizations through the activation of triple bonds.
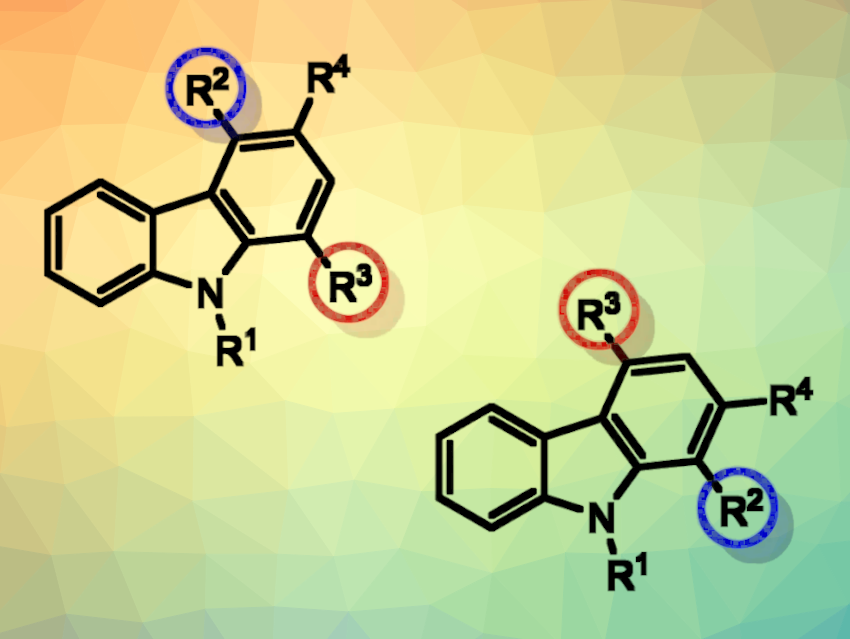
Roberto Sanz, University of Burgos, Spain, and colleagues have developed a regiodivergent synthesis of highly substituted carbazoles through a gold-catalyzed cyclization of alkynols bearing an indol-3-yl and an additional group at the homopropargylic positions (pictured below) under mild reaction conditions. This protocol involves the activation of these substrates to form a spirocyclic intermediate, which can further react via two different cyclization pathways.
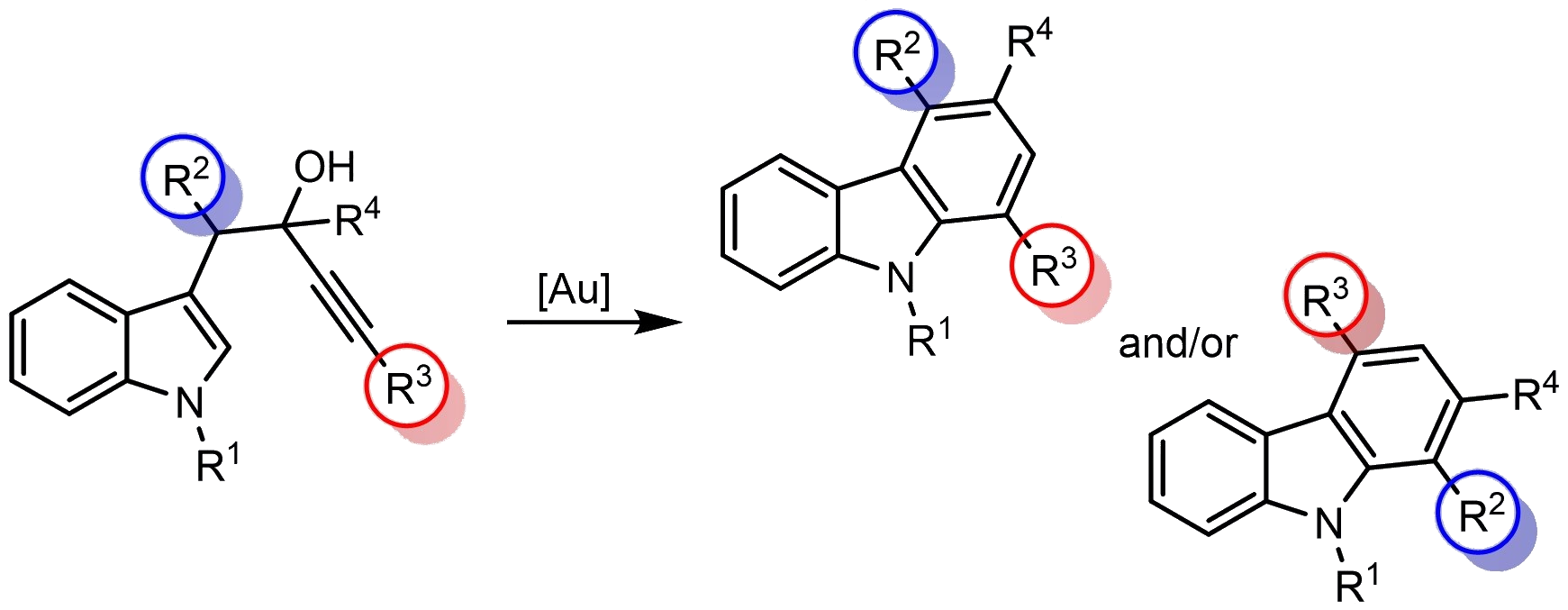
The regioselectivity of the reaction can be controlled by the oxidation state of the gold catalyst as well as the electronic nature of the substituents of the alkynol unit. With gold(I) complexes and for electron-rich aromatic substituents at the homopropargylic position, a 1,2-alkyl migration is favored in the spiroindoleninium intermediate. When using gold(III) salts and for alkyl groups or non-electron-rich aromatic groups, a 1,2-alkenyl shift is preferred.
The two pathways lead to two different isomers of the highly substituted carbazole products. The method has a wide substrate scope, giving a variety of carbazoles with good yields and regioselectivities.
- Catalyst‐ and Substrate‐Controlled Regiodivergent Synthesis of Carbazoles through Gold‐Catalyzed Cyclizations of Indole‐Functionalized Alkynols,
Marta Solas, Miguel A. Muñoz-Torres, Fernando Martínez-Lara, Lorena Renedo, Samuel Suárez-Pantiga, Roberto Sanz,
ChemPlusChem 2023.
https://doi.org/10.1002/cplu.202300382