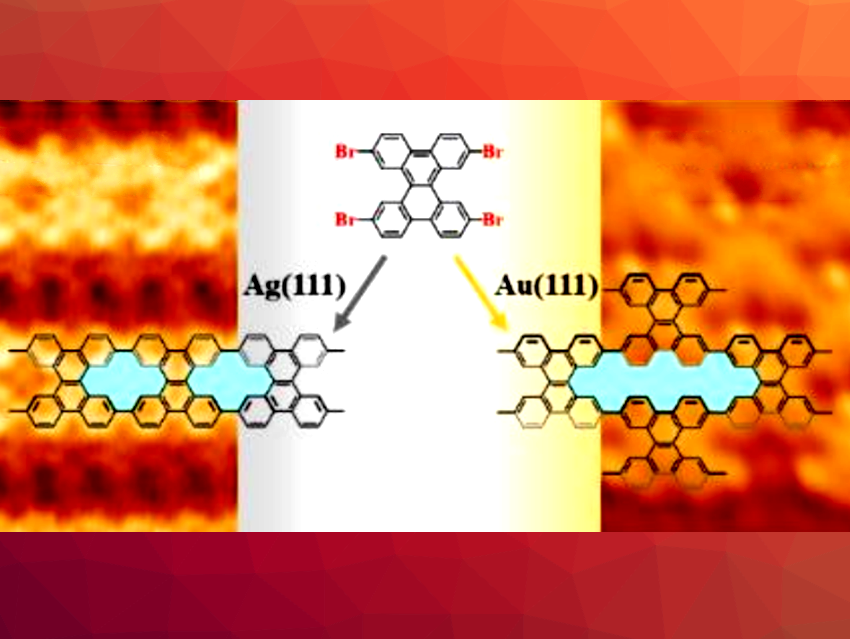
Graphene-based nanomaterials can be useful in a variety of applications. Their electronic and mechanical properties can be tuned, e.g., by introducing nanopores into their structures. On-surface synthesis can be used for the synthesis of porous, low-dimensional, graphene-based nanostructures with atomic precision. So far, this approach has been used to create various different types of graphene nanostructures containing planar [18]annulene pores on metal surfaces. The synthesis of graphene structures containing nonplanar [14]annulene pores has been more challenging due to the steric hindrance between adjacent hydrogen atoms inside the pores.
Dezhou Guo, Beijing Institute of Technology, China, Tao Wang, Donostia International Physics Center, San Sebastián, Spain, Junfa Zhu, University of Science and Technology of China, Hefei, and colleagues have developed a method for the selective synthesis of two different graphene-based nanostructures, containing either [14]annulene or [30]annulene pores, on different metal substrates (pictured). Both syntheses start from the same precursor, namely, 2,7,10,15-tetrabromodibenzo[a,c]triphenylene (TBDBTP).
Distinct Products on Different Substrates
When the precursor was deposited on an Ag(111) surface at 410 K, the researchers observed the formation of linear porous organometallic polymers. Further annealing at 510 K led to a release of the silver atoms in the structure and gave porous graphene nanoribbons containing periodic [14]annulene pores. This was observed using high-resolution scanning tunneling microscopy (STM).
When the same precursor was deposited on an Au(111) surface, staggered, chain-like structures were formed at 450 K. In this case, further annealing at 510 K led to the formation of porous graphene nanosheets with periodic [30]annulene pores.
The team proposes that the difference in products originates from the competition between the debromination of TBDBTP and C−C coupling. On Ag(111), the activation temperature of TBDBTP debromination is lower than that of the coupling reaction, leading to the formation of organometallic motifs in the first step. On Au(111), a coupling reaction of TBDBTP occurs immediately after debromination, generating staggered covalent chains after partial debromination, which fuse upon further annealing.
- Synthesis of a Porous [14]Annulene Graphene Nanoribbon and a Porous [30]Annulene Graphene Nanosheet on Metal Surfaces,
Tianchen Qin, Dezhou Guo, Juanjuan Xiong, Xingyu Li, Lei Hu, Weishan Yang, Zijie Chen, Yulun Wu, Honghe Ding, Jun Hu, Qian Xu, Tao Wang, Junfa Zhu,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202306368