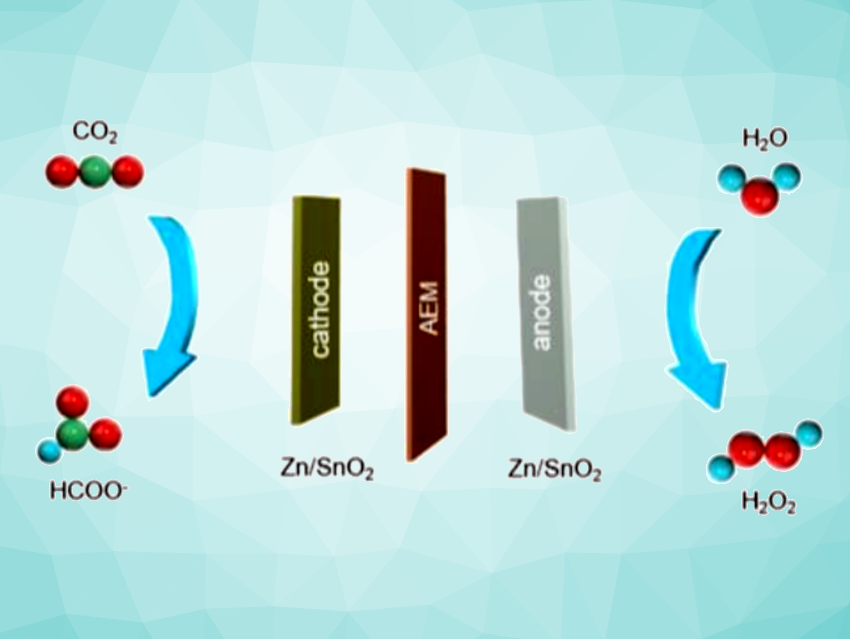
Hydrogen peroxide (H2O2) and formate (HCOO–) are important chemicals used in various industries. The two-electron water oxidation reaction (WOR) can convert H2O into H2O2, and formate can be produced via the electrocatalytic reduction of CO2. One promising approach to the simultaneous production of these two chemicals is the coupling of these two reactions in an electrolyzer using bifunctional electrocatalysts.
Jinlong Liu, Central South University, Hunan, China, Bao Yu Xia, Bo You, Huazhong University of Science and Technology, Hubei, China, and colleagues have developed a hybrid electrosynthesis strategy for the synthesis of H2O2 and formate, using Zn-doped SnO2 (Zn/SnO2) nanodots as bifunctional redox electrocatalysts. The team prepared the nanodots from SnCl4·5H2O and Zn(Ac)2 via a simple hydrothermal method. They were then used in an anion exchange membrane (AEM)-separated two-chamber flow electrolyzer with Zn/SnO2 as both the anode and cathode electrocatalyst.
Using this system, the team achieved Faradaic efficiencies of 80.6 % and 92.2 % for H2O2 and formate coproduction. They also observed excellent stability for at least 60 h at a current density of ~150 mA cm–2. According to the researchers, the Zn dopant promotes the coupling of *OH intermediates, which improves H2O2 production at the anode. It also optimizes the adsorption of *OCHO intermediates, which supports the synthesis of formate at the cathode.
- Simultaneous Generation of H2O2 and Formate by Co‐Electrolysis of Water and CO2 over Bifunctional Zn/SnO2 Nanodots,
Xin Hu, Guoliang Mei, Xiangxiong Chen, Jinlong Liu, Bao Yu Xia, Bo You,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202304050