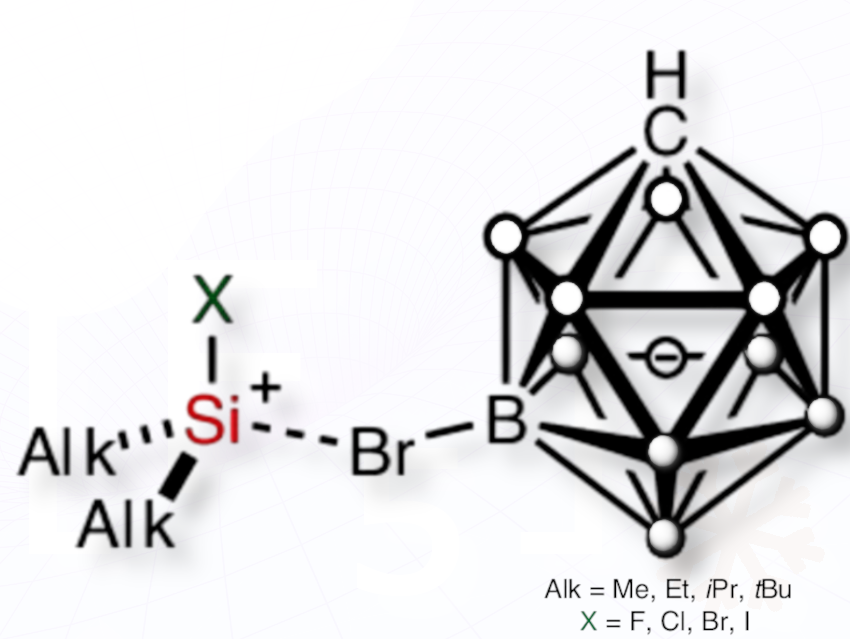
Martin Kaupp, Hendrik F. T. Klare, Martin Oestreich, and colleagues at Technische Universität Berlin, Germany, have synthesized and characterized all halogen-substituted silylium ions of the type [Alk₂XSi(HCB₁₁H₅Br₆)] (X = F, Cl, Br, or I; Alk = Me, Et, iPr, or tBu)—long-sought, highly reactive silicon-based species.
The halogen-substituted silylium ions were synthesized via protolysis of halosilanes (Alk₂XSi–LG), where LG = H or Ph, using Reed’s superacidic benzenium salt H(C₆H₆)⁺[HCB₁₁H₅Br₆]⁻. This dehydrogenative (LG = H) or dephenylative (LG = Ph) approach provided access to the full series of these ions. This route was necessary because the conventional Corey hydride transfer reaction failed to produce them in the condensed phase.
Stabilized with a special carborate counterion, these ions are among the strongest Lewis acids ever isolated. These superacids could enable new catalytic strategies and play crucial roles in waste recycling (e.g., the Müller–Rochow process) and the breakdown of persistent pollutants like PFAS.
- Isolation of halogen-substituted silylium ions
Tobias Randt, Morten Lehmann, Elisabeth Irran, Martin Kaupp, Hendrik F. T. Klare, Martin Oestreich
Nature Chemistry 2025
https://doi.org/10.1038/s41557-025-01880-2