Hexagonal boron nitride (h-BN) is a material that is structurally similar to graphite. It is chemically and thermally stable and mechanically robust. h-BN can be employed, e.g., in catalyst supports, corrosion inhibitors, solid lubricants, or as a reinforcing phase for polymers.
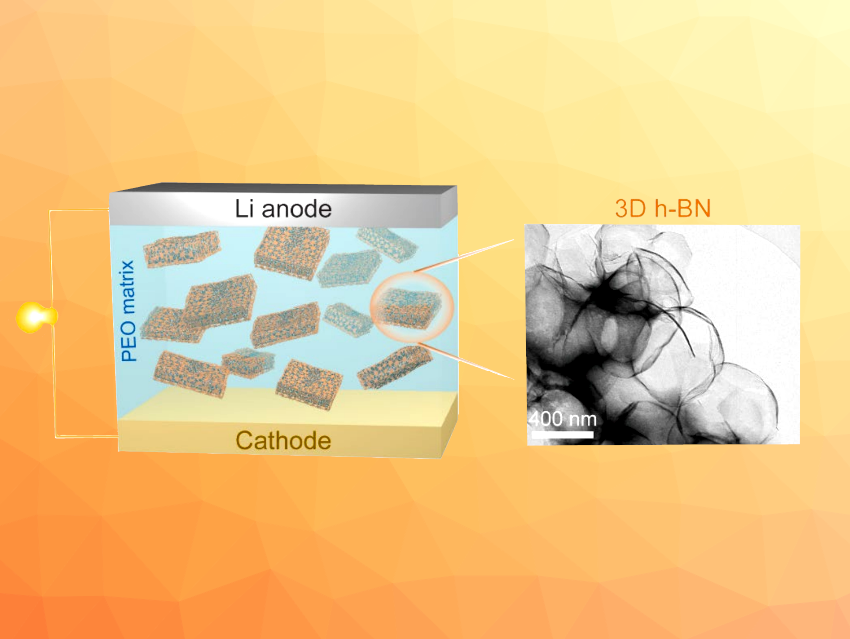
Two-dimensional h-BN nanosheets have been used, for example, to improve the performance of solid-state electrolytes in lithium metal batteries, where they can enhance the mechanical strength and improve the electrolyte’s ionic conductivity due to Lewis acid-base interactions between h-BN and anions. 2D-structured materials, however, can aggregate in the polymer matrix, which leads to an uneven distribution. h-BN with a three-dimensional structure could, thus, further improve performance. However, no method for the synthesis of powdery h-BN with a 3D structure had been reported so far.
Chunnian He, Joint School of the National University of Singapore and Tianjin University, Fuzhou, China, Tianjin University, China, Andrew Barnabas Wong, National University of Singapore, and colleagues have developed a strategy to synthesize 3D h-BN with a micron-sized framework that has a honeycomb-like structure, using a simple NaCl/glucose-assisted method. The team’s process involves three steps: 1) freeze-drying an aqueous solution containing NaCl, glucose, and boric acid, 2) heating the freeze-dried powder to 750 °C under a flow of Ar and NH3, and 3) removing NaCl by washing and residual carbon by calcination at 600 °C.
According to the researchers, the NaCl facilitates the nitridation reaction of oxygen-containing boron compounds and acts as a 3D template for h-BN. The glucose contributes to the uniform distribution of boric acid onto the NaCl surface and plays a role as a reductant. The prepared 3D h-BN was used as a filler in a polyethylene oxide (PEO)-based solid-state electrolyte. The resulting electrolyte showed enhanced ionic conductivity and mechanical strength.
- The Synthesis of Three‐Dimensional Hexagonal Boron Nitride as the Reinforcing Phase of Polymer‐Based Electrolyte for All‐Solid‐State Li Metal Batteries,
Yuhan Ma, Jiaxin Wu, Haonan Xie, Rui Zhang, Yiming Zhang, Enzuo Liu, Naiqin Zhao, Chunnian He, Andrew Wong,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202317256