He Li, Xi’an Jiaotong University, China, discusses using amorphous-like copper–zinc atomic clusters on silicon nanowires to enable faster, more efficient urea formation.
Urea is a key nitrogen fertilizer essential for global agriculture. It also has industrial applications in resins, plastics, and pharmaceuticals. Traditional urea production relies on the Haber–Bosch process followed by reaction with CO₂, which requires high temperatures and pressures and consumes large amounts of energy. Previous photoelectrochemical approaches struggled with low selectivity and efficiency because controlling the reactive nitrogen and carbon intermediates (*NH₂, *NO₂, *CO) was difficult, often leading to by-products like NH₃ or N₂H₄.
What did you do?
We have developed a novel class of photocathodes, which integrates copper–zinc amorphous-like ultra-small atomic clusters and silicon nanowire arrays. This photocathode enables highly efficient photoelectrochemical synthesis of urea from carbon dioxide (CO₂) and nitrate (NO₃⁻) under ambient conditions, using simulated one-sun illumination and minimal electrical energy to produce urea, a vital nitrogen fertilizer.
Why are you interested in this?
The industrial production of urea is an energy-intensive process. Using ambient conditions to make urea from CO₂ and nitrate is a much more sustainable and cleaner strategy.
However, this reaction is very complex and inefficient with existing catalysts. We aim to design a better catalyst to overcome these inefficiencies and make the process more efficient.
What is new and cool about your work?
The new and cool part about this work is the “oxidation state inversion” in the catalyst.
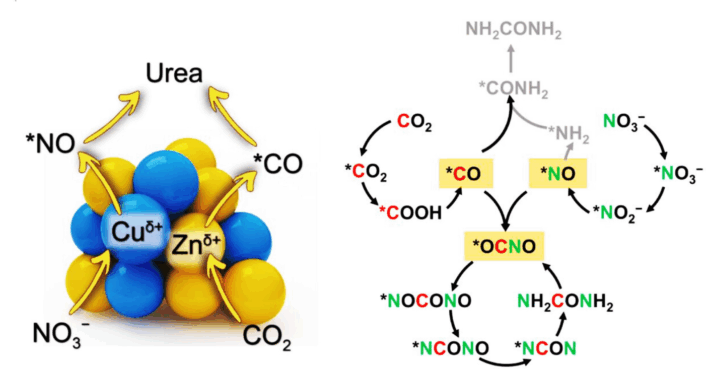
In conventional crystalline materials, copper and zinc show constrained oxidation states that limit their catalytic efficiency toward urea synthesis. By contrast, the designed amorphous-like atomic clusters broke this inherent oxidation state constraint, leading to copper species with higher oxidation states and zinc species with lower oxidation states.
This oxidation state inversion results in highly asymmetric active sites, where the electron-deficient copper sites selectively adsorb and reduce nitrate to form the critical *NO intermediate, while the electron-rich zinc sites promote CO₂ reduction to *CO. The *NO and *CO intermediates significantly reduce the energy barrier required for the crucial carbon-nitrogen (C-N) bond formation step, enabling highly efficient urea formation.
What are your key findings?
The key finding of this work is that designing a catalyst with an amorphous-like atomic structure enables precise modulation of the oxidation state at the metal sites. This allows the reaction to follow a new and highly efficient pathway centered around the *NO intermediate, rather than competing routes that typically produce undesirable by-products such as ammonia and hydrazine. As a result, the process achieves a highly efficient and selective process in urea synthesis.
What specific applications do you imagine?
This study opens up pathways to several applications, as explained below, with the longer-term vision centered on sustainable chemical synthesis and environmental remediation.
Catalyst Design Principle: The fundamental principle of using amorphous engineering to tailor oxidation states in bimetallic catalysts can be extended to design high-performance catalysts for other complex reactions involving multi-electron transfer, such as CO₂ hydrogenation or nitrogen fixation, greatly broadening its impact in green chemistry and energy conversion.
Sustainable Agriculture: Beyond the immediate goal of urea production, the technology will enable on-site, solar-driven fertilizer generation using CO₂ from air and nitrate from wastewater, reducing reliance on energy-intensive industrial processes.
Carbon and Nitrogen Utilization: This research offers a promising strategy for closing global carbon and nitrogen cycles by converting waste pollutants into valuable products.
What part of your work was the most challenging?
The most challenging part of this work is unequivocally proving the existence and role of the oxidation state inversion and the resulting asymmetric atomic structure within the amorphous-like copper–zinc ultra-small atomic clusters.
We had to synthesize the material with consistent conditions and extreme precision, and then employ advanced characterization techniques, including X-ray photoelectron spectroscopy (XPS), X-ray absorption fine structure (XAFS) analysis, and high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM), to probe the oxidation state and coordination environment of Cu and Zn. This process was further complemented by ab initio molecular dynamics simulations to model the amorphous-like structure.
Correlating the results from these diverse methods to build a definitive mechanism of the oxidation state inversion posed a significant experimental and analytical challenge, which was essential to substantiate our proposed mechanism.
What achievement in this study are you most proud of?
This work not only demonstrates a practical route for sustainable urea production but also establishes a new design principle in catalysts, leveraging amorphous-like structure to break oxidation state constraints and activate unconventional reaction pathways. This suggests that strategically engineered disorder can be a powerful tool for tailoring activity and selectivity in multi-step reactions.
This approach could inspire future efforts in CO₂ conversion, nitrogen fixation, and other complex electrocatalytic processes where dynamic active sites and flexible electronic environments are advantageous.
Thank you very much for sharing these insights.
The paper they talked about:
- Efficient C−N Coupling via Oxidation State Inversion in Asymmetric Copper–Zinc Amorphous-Like Atomic Clusters Driving Photoelectrocatalytic Urea Synthesis,
Yuxiu Zou, Xiangjiao Gong, Wenli Zhao, Xiaxin Wang, Chaoqun Jia, Honghui Ou, Bo Lin, He Li, Dingsheng Wang, Guidong Yang,
Angewandte Chemie 2025.
https://doi.org/10.1002/anie.202517559
He Li is a professor at the State Key Laboratory of Fluorine and Nitrogen Chemicals in the School of Chemical Engineering and Technology at Xi’an Jiaotong University in Shaanxi, China.
Also of Interest