Urea is a key component of fertiliser, and its large-scale production is therefore critical to the agriculture industry. Its standard industrial synthesis follows the Bosch–Meiser process, which involves high-temperature, high-pressure reactions to convert ammonia and carbon dioxide into ammonium carbamate, followed by decomposition of the carbamate to generate urea.
The electrocatalytic co-reduction of NO₃⁻ and CO₂ may present a greener, more sustainable method to replace this energy-intensive process, but it has proven challenging due to a lack of selectivity and competing reactions.
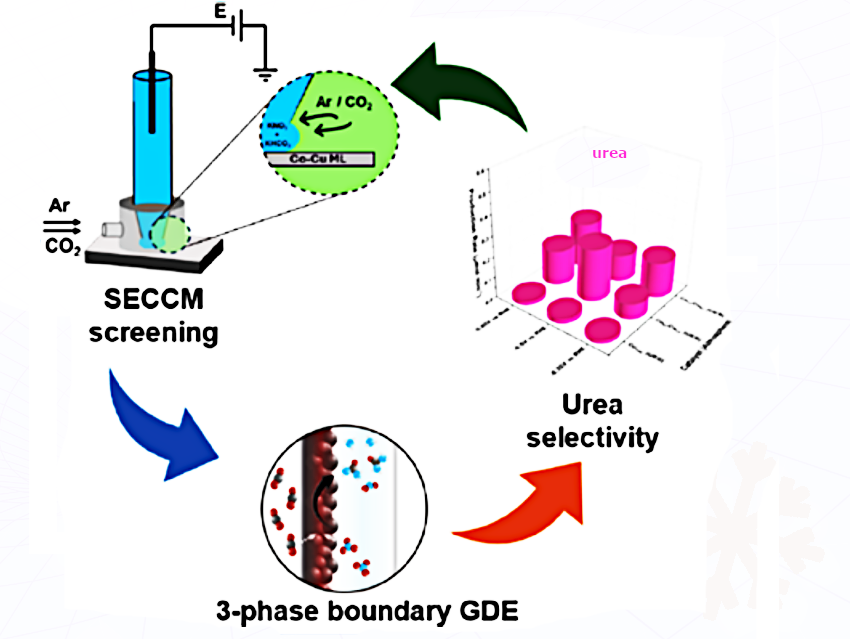
A team at Ruhr University Bochum in Germany and the Shanghai Advanced Research Institute, Chinese Academy of Sciences, China, has found a way to speed things up: they created a library of CoCu thin film materials and used scanning electrochemical cell microscopy to determine the composition-dependent electrochemical activity of each.
This high-throughput method used an electrolyte containing NO₃⁻ and compared the overpotentials of each sample with and without CO₂, identifying matching potentials for the two necessary reactions. The optimal composition could then be tested for urea production in a model flow-through electrolyzer.
Given the high level of academic and industrial interest in more efficient and selective catalytic processes, rational design strategies such as this can lead big long-term gains, making not only the resulting materials and processes but also the research process itself more sustainable.
- Co-Cu Materials Library Screening for Discovering Electrocatalysts for the Co-Reduction of CO₂ and Nitrate to Urea
Jialin Shi, Geovane Arruda de Oliveira, Xin Wang, Jian Zhang, Ieva Cechanaviciute, Jill Fortmann, Alfred Ludwig, Wolfgang Schuhmann
ChemistryEurope 2025
https://doi.org/10.1002/ceur.202500333