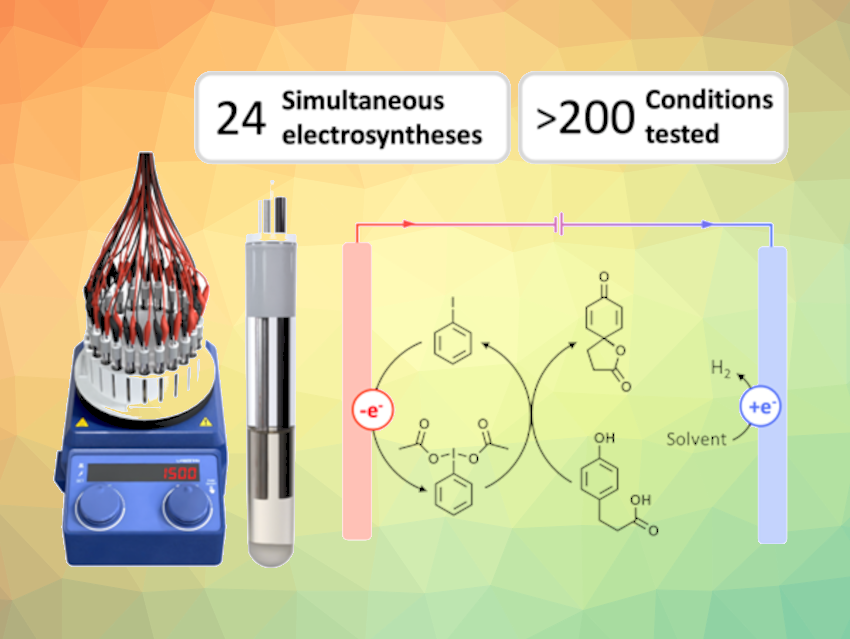
Organic chemists familiar with electrosynthesis will likely share the sentiment: Optimizing reactions in organic electrochemistry can be tedious and overwhelming. Many parameters unique to electrochemistry need to be investigated, including the choice of materials for the electrodes, the optimal electrolyte, the duration of electrolysis, etc. The impact of these parameters is often not fully explored due to the vast number of experiments required.
Eric McCalla, Janine Mauzeroll, McGill University, Montréal, Québec, Canada, and colleagues have introduced an electrochemical reactor designed for high-throughput exploration (design pictured below). This device runs up to 24 individually controlled electrosynthetic cells simultaneously, providing researchers with a platform to test hundreds of reaction conditions in a matter of weeks. The available methods include constant potential, constant power, pulsed techniques, and custom combinations.

With over 200 individual syntheses under different conditions, the researchers performed a deep dive into the case of the hypervalent-iodine-mediated oxidation of phloretic acid using their reactor. This case study demonstrated the effects of multiple parameters on yield, but also on reproducibility, through systematic optimization.
For a total cost of around USD $8,200, the developed device could offer a cost-effective option for academic or industrial laboratories that want to explore the complex field of electrochemistry.
- Overcoming Challenges in Electrosynthesis Using High‐Throughput Electrochemistry: Hypervalent Iodine‐Mediated Phenol Dearomatization, a Case Study,
Antoine Juneau, Marzieh Abdolhosseini, Camille Rocq, Hanh D. M. Pham, Mia Pascall, Rustam Z. Khaliullin, Sylvain Canesi, Eric McCalla, Janine Mauzeroll,
ChemElectroChem 2024.
https://doi.org/10.1002/celc.202400193