Polar fluorinated arenes such as trifluorotoluene, fluorobenzene, 1,2-difluorobenzene, etc., can be useful in organic synthesis and as fluorous solvents. Free radical reactions can be promoted by these solvents. Thus, understanding the interactions between organic radicals and these solvents is an interesting research objective.
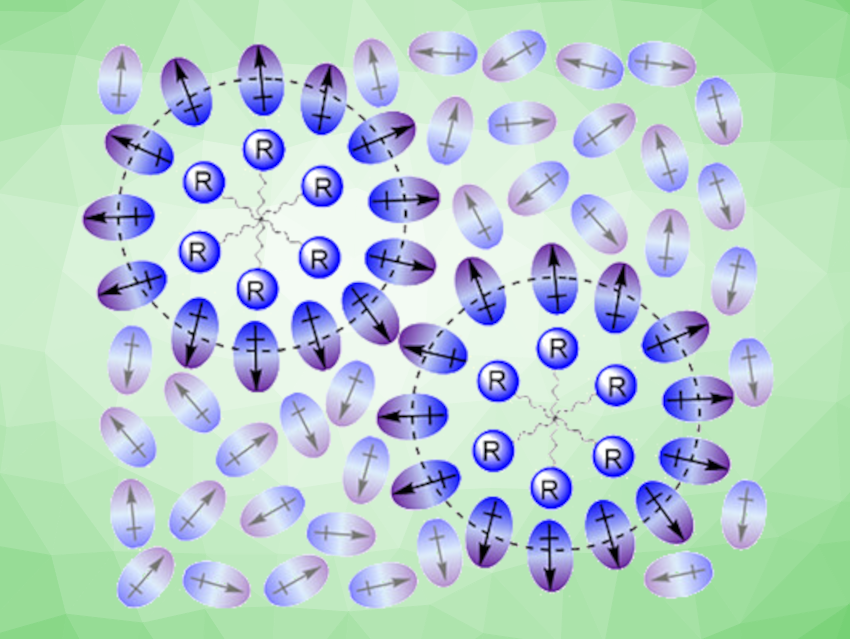
Tianfei Liu, Nankai University, Tianjin, China, and colleagues have investigated the aggregation of organic free radicals in five polar fluorinated arenes as solvents and analyzed the influence of the radicals’ substituents on the aggregation. The team considered radicals such as (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) as a simple radical, 4-carboxy-TEMPO as a radical that can form hydrogen bonds, or methyl-5-doxyl-stearate as a radical with a long, saturated hydrocarbon chain substituent. The aggregation of these radicals was studied in the solvents fluorobenzene, 1,2-difluorobenzene, 1,2,3-trifluorobenzene, trifluorotoluene, and 2-fluoro-substituted trifluorotoluene. The researchers used UV-vis spectroscopy, transmission electron microscopy (TEM), electron paramagnetic resonance (EPR) spectroscopy, and electrochemical experiments to investigate the radicals’ aggregation behavior in these solvents and find, e.g., the critical aggregation concentration and the shapes and sizes of the aggregates.
The team found that in these solvents, small organic radicals with simple substituents form a homogeneous solution. In contrast, radicals with substituents that form intermolecular hydrogen bonds or radicals with long aliphatic hydrocarbon chains tend to aggregate. They also observed that the aggregation of radicals with intermolecular hydrogen bonds can significantly increase the rate of the free-radical-catalyzed electro-oxidation of benzyl alcohol in this type of solvent. The team proposes an “aggregate shuttle” mechanism that explains this effect, in which aggregates transport reactants through the diffusion layer to the electrode surface and products back to the bulk solution.
- The aggregation behaviors of organic radicals in polar fluorinated arenes,
Shan Liu, Yin Yang, Tianfei Liu,
Aggregate 2024.
https://doi.org/10.1002/agt2.543