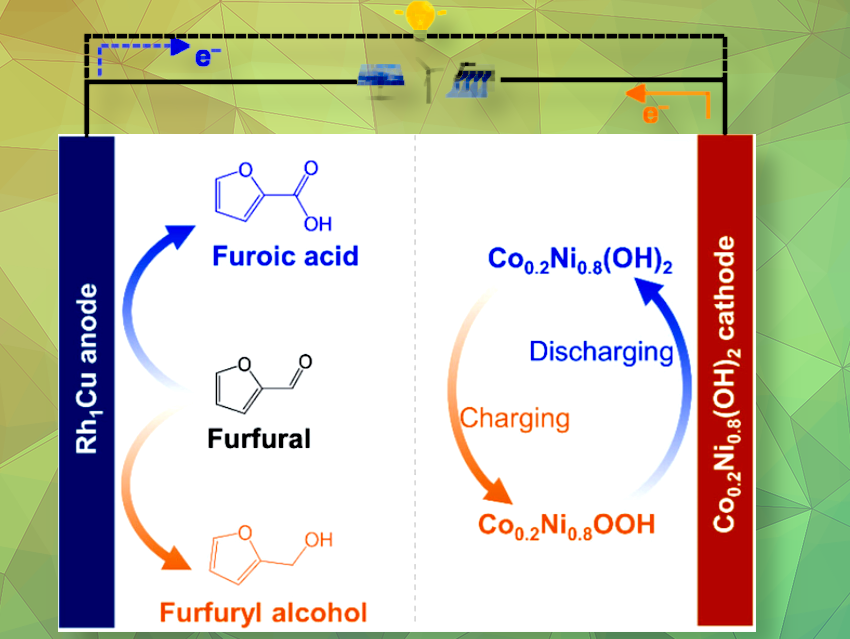
Rechargeable batteries store electricity in their electrode materials, while redox flow batteries use chemicals stored in tanks attached to the electrodes. Researchers have developed a battery system based on a hybrid cell, which not only stores and provides electricity but also produces valuable chemicals in a flow system. During operation, the furfural–nickel hydroxide battery converts biomass-derived molecular furfural into either furfuryl alcohol or furoic acid.
Furfural–Nickel Hydroxide Battery
Furfural is a small molecule formed from pentose sugars common in agricultural biomass, and it is considered an important platform chemical from which a number of intermediates can be obtained for various applications. It can be oxidized to furoic acid, a food preservative and intermediate in the synthesis of pharmaceuticals and fragrances. When reduced, furfural is converted into furfuryl alcohol, a precursor in resins, flavors, and drugs. Haohong Duanand colleagues, Tsinghua University, Beijing, China, have succeeded in obtaining both value-added chemicals during the operation of a hybrid flow battery, increasing the cost efficiency of the battery system.
When charged, standard rechargeable batteries store electricity in their electrodes and feed it into a circuit as they discharge. Another battery type, redox flow batteries, store electricity in chemicals, with the chemical products cycling between two states and remaining within the battery. Combining both concepts, the researchers investigated the extent to which such batteries are able to produce extra chemicals while storing or providing energy.
Bifunctional Metal Catalyst for the Anode
A breakthrough came in the form of a bifunctional metal catalyst for the anode. Made of a rhodium–copper (Rh1Cu) single-atom alloy, this catalyst smoothly converted electrolyte-containing furfural into furfuryl alcohol when the battery was charged, while furoic acid was formed as the battery was discharged. For the cathode, the researchers identified a cobalt-doped nickel hydroxide material (Co0.2Ni0.8(OH)2), similar to cathode materials used in traditional nickel–zinc or nickel–metal hydride batteries.
This assembly led to a true dual-use battery system: after charging (using a solar cell), four series-connected hybrid batteries were able to run various devices, including LED lights and smart phones, while continually producing furfuryl alcohol and furoic acid during battery cycling, with these chemicals being conducted away using a flow system.
Energy and Power Density
The researchers found that the new hybrid battery is comparable to a number of common batteries in terms of energy density and power density, but it provides both power and value-added chemicals at the same time. While storing 1 kWh of energy, 0.7 kg of furfuryl alcohol is produced, and 1 kg of furoic acid is produced when the system provides a power of 0.5 kWh (on which a refrigerator can run for a couple of hours). However, furfural is continually fed into the system and the products must be separated from the electrolyte.
The team’s hybrid concept is a step toward improving the sustainability and cost effectiveness of rechargeable batteries, but there is still a need to develop the concept further.
Reference
- Rechargeable Biomass Battery for Electricity Storage/Generation and Concurrent Valuable Chemicals Production,
Jing Li, Kaiyue Ji, Boyang Li, Ming Xu, Ye Wang, Hua Zhou, Qiujin Shi, Haohong Duan,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202304852