Perfluoroalkyl compounds are persistent pollutants that can be harmful to the environment. They can, for example, contribute to global warming or accumulate in living organisms. Due to the strength of the C(sp3)–F bonds, perfluoroalkyl substances can have high chemical and thermal stabilities, which can make it difficult to convert them to less harmful species. Hydrodefluorination, i.e., replacing fluorine atoms with hydrogen atoms, for example, can provide a way to dispose of perfluoroalkyl compounds. However, existing hydrodefluorination reactions are often limited to the conversion of C(sp2)–F bonds.
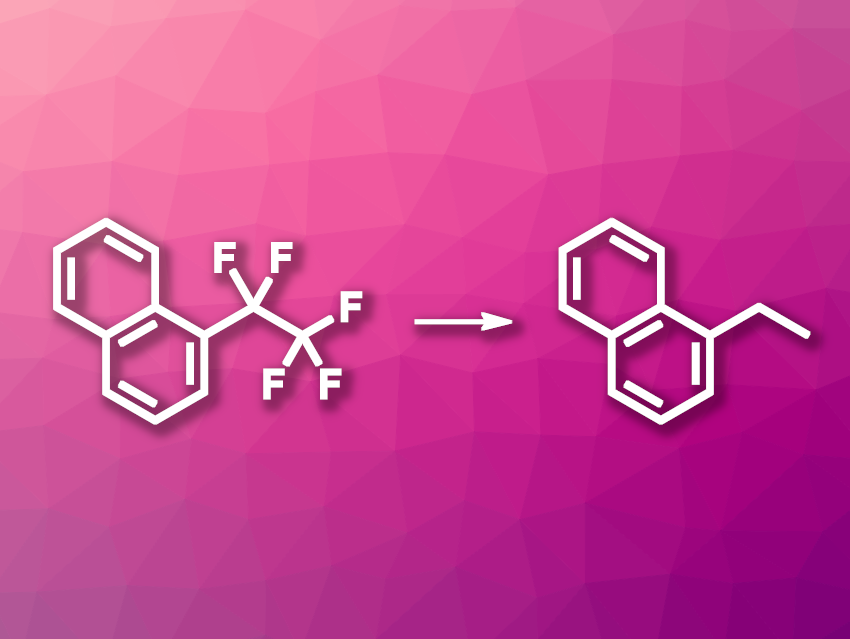
Ryohei Doi, Sensuke Ogoshi, Osaka University, Japan, and colleagues have developed a method for the exhaustive hydrodefluorination of perfluoroalkyl arenes (example reaction pictured)—i.e., for replacing all fluorine atoms in perfluoroalkyl arenes with hydrogen. The team used bis(cyclooctadiene)nickel(0) (Ni(cod)2) as the catalyst together with 1,3-dicyclohexyllimidazol-2-ylidene (ICy) as an N-heterocyclic (NHC) ligand. Using this catalyst system, they reacted perfluoroalkyl arenes such as 1-pentafluoroethylnaphthalene, 1-heptafluoropropylnaphthalene, or 1-nonafluorobutylnaphthalene with Me2PhSiH in the presence of K3PO4 as a base, using dimethylformamide (DMF) as the solvent.
Using this approach, the researchers obtained the desired exhaustive hydrodefluorination products in yields of up to 86 % (for 1-ethylnaphthalene). Substrates with longer perfluoroalkyl chains gave much lower yields, but small amounts of the respective fully hydrodefluorinated products were still detected. The team proposes a reaction sequence that involves a hydrodefluorination at the benzylic position as the first step, followed by a nickel-promoted elimination of HF to give a difluroalkene. The next step is a C(sp2)–F bond hydrodefluorination to give an alkene, which undergoes hydrosilylation, Finally, protonation releases the desired product.
- Nickel-Catalyzed Exhaustive Hydrodefluorination of Perfluoroalkyl Arenes,
Ryohei Doi, Masashi Yasuda, Naoki Kajita, Kenta Koh, Sensuke Ogoshi,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c03471