Subhash Garhwal, Massachusetts Institute of Technology (MIT), Cambridge, MA, USA, together with colleagues has developed a highly enantioselective method for the hydroformylation of vinyl arenes that uses copper hydride catalysis and zinc triflate and yields enantioenriched α-aryl acetal products. These products can be converted to aldehydes, alcohols, and amines while maintaining enantiomeric purity.
Can you give a summary of what you describe in the article?
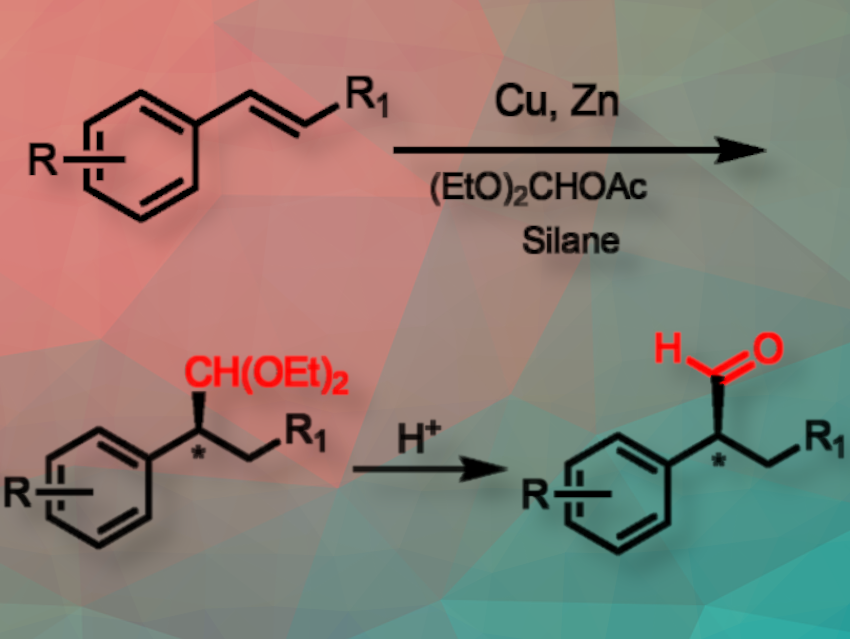
In this article, we report the development of a first base metal copper hydride-catalyzed protocol for the enantioselective net hydroformylation of vinyl arenes. This method provides access to α-aryl acetal and aldehyde derivatives with high levels of enantioselectivity and tolerance of various functional groups and heterocycles.
We show that the acetal products could be converted to the corresponding aldehydes, alcohols, and amines with full preservation of the enantiomeric purity. Computational studies suggest a crucial role of the catalytic Lewis acid zinc (II) triflate in the activation of the orthoester electrophile.
Additionally, using commercially available diethyl methyl acetate as a C1 source sidesteps the need for high-pressure CO gas. In this way, the problems that have hindered the development of base metal-catalyzed asymmetric hydroformylation can also be avoided.
Why are you doing this?
Enantiomerically enriched aldehydes are versatile synthetic intermediates and could be converted into various useful molecules such as alcohols, amines, and acids, such as fenoprofen and ibuprofen, with preservation of enantioselectivity, as we have shown in the article.
Hydroformylation, also known as oxo synthesis, is a chemical reaction in which carbon monoxide and hydrogen are added to an unsaturated compound, typically an alkene or alkyne. The hydroformylation of olefins is the most widely used homogenous catalytic process, as it is an atom-economical and cost-effective strategy for the synthesis of aldehydes.
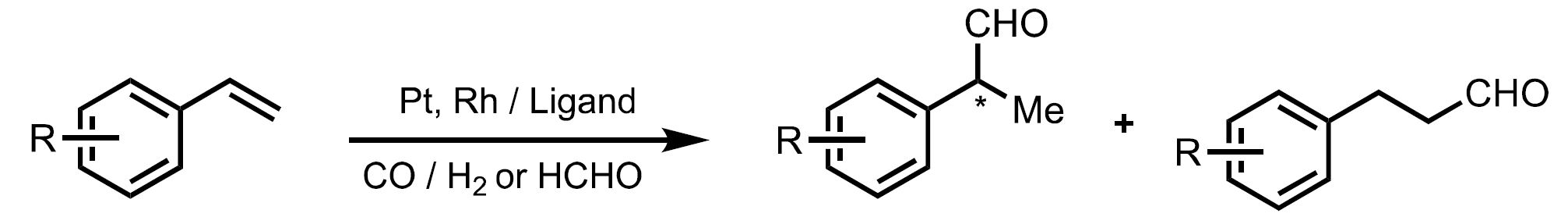
Figure. Precious metal-catalyzed asymmetric hydroformylation.
The development of base metal-catalyzed enantioselective hydroformylation has been inhibited by the inherent challenge of regioselectivity (branched vs. linear aldehyde products) and chemoselectivity (hydroformylation vs. alkene or aldehyde hydrogenation) in addition to the formation of new stereogenic centers. Base metal catalysts will provide a cheaper and more sustainable alternative to precious metals in asymmetric hydroformylation. This reaction is important for the production of chiral aldehydes used in pharmaceuticals and fragrances.
What is new and cool about it?
This is the first report using a base metal—a first-row transition metal that is abundant and less toxic—to perform asymmetric formal hydroformylation. This method shows excellent enantio- and regio-selectivity as precious metals.
The products were also acetals (protected aldehydes), which allowed the isolation of products without racemization. This method also utilized commercially available diethylmethyl acetate as the C1 source and avoided the use of toxic CO gas.
What are your key findings?
Copper hydride (CuH) catalysis is used to enable highly enantioselective formal hydroformylation of vinyl arenes. Key to the success of this method is the use of zinc triflate to activate diethoxymethyl acetate, which is used as the formyl source. This protocol allowed efficient hydroacetalization of a wide range of vinyl arene substrates to form highly enantioenriched α-aryl acetal products with excellent regioselectivity and good yields.
As mentioned above, these acetal products could be converted into a variety of enantiomerically enriched products such as alcohols, amines, and acids (including the drug molecules fenoprofen and ibuprofen) with preservation of enantioselectivity.
In collaboration with the group of Professor Peng Liu at the University of Pittsburgh, USA, we investigated the reaction mechanism using density functional theory and found out the role of the catalytic Lewis acid zinc(II) triflate in the activation of the orthoester electrophile to form a highly reactive oxocarbenium ion. DFT studies also suggested the key stereo-inversion C-C bond-forming event between the stereo-enriched alkyl copper intermediate and the oxocarbenium electrophile in a backside SE2-type mechanism.
What specific applications do you imagine?
In industrial organic chemistry, transition metal-catalyzed hydroformylation is an important homogeneously catalyzed reaction. Millions of tons of aldehydes and related chemicals are produced annually.
However, most processes use precious rhodium catalysts due to their high efficiency. Our long-term goal is to develop a general strategy for the use of base metals in the asymmetric hydroformylation of various unsaturated feedstocks.
What was the most challenging part of your work?
Compared to established precious metal catalysts in asymmetric hydroformylation, base metal alternatives face several intrinsic challenges. Controlling regioselectivity and chemoselectivity is more challenging, with base metal catalysts showing a propensity for unwanted hydrogenation and isomerization reactions.
To overcome these challenges, commercially available diethylmethyl acetate is used as the C1 source, and an active electrophile was generated in situ using a catalytic amount of zinc(II) triflate to mitigate side reactions between oxocarbenium and the hydride source.
In addition, the products obtained in this process were acetals, which helped prevent over-reduction of the desired aldehydes to alcohols, a common side reaction in traditional base metal-catalyzed hydroformylation. Overcoming these challenges holds significant potential due to the inherent abundance and cost-effectiveness of base metals compared to precious metals.
What are your hopes for the future?
These findings will open new avenues for the use of base metals in the development of asymmetric hydroformylation of various unsaturated substrates.
Thank you for the insights.
The article they talked about:
- CuH-Catalyzed Regio- and Enantioselective Formal Hydroformylation of Vinyl Arenes,
Subhash Garhwal, Yuyang Dong, Binh Khanh Mai, Peng Liu, Stephen L. Buchwald,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c04287
Subhash Garhwal is a post-doctoral associate at the Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA, USA, in the group of Stephen L. Buchwald.