The reaction of alkynes with azides, a representative Huisgen reaction, is useful to generate 1,2,3-triazoles. Copper-catalyzed reactions of terminal acetylenes with azides at room temperature to give 1,4-disubstituted 1,2,3-triazoles are typical reactions in the widely used “click” chemistry. Extending this type of reaction to other types of multiple bonds would be interesting. For example, a variety of B−B multiply bonded species are known, but cycloadditions with 1,3-dipolars such as azides had not yet been achieved.
Lizhao Zhu and Rei Kinjo, Nanyang Technological University, Singapore, have found that a Huisgen cycloaddition involving a B–B unsaturated bond is possible. The team first synthesized a 1,2-diboraallene via the reduction of a cyclic (alkyl)(amino)carbene (CAAC) and trimethylphosphine-coordinated tetrabromodiborane with KC8 in the presence of PMe3. Then they reacted the 1,2-diboraallene with the bulky 2,6-diisopropylphenyl azide under catalyst-free and mild conditions to produce a diboratriazole (pictured).
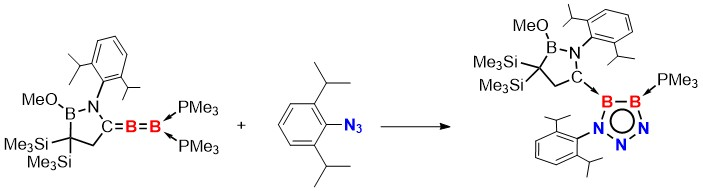
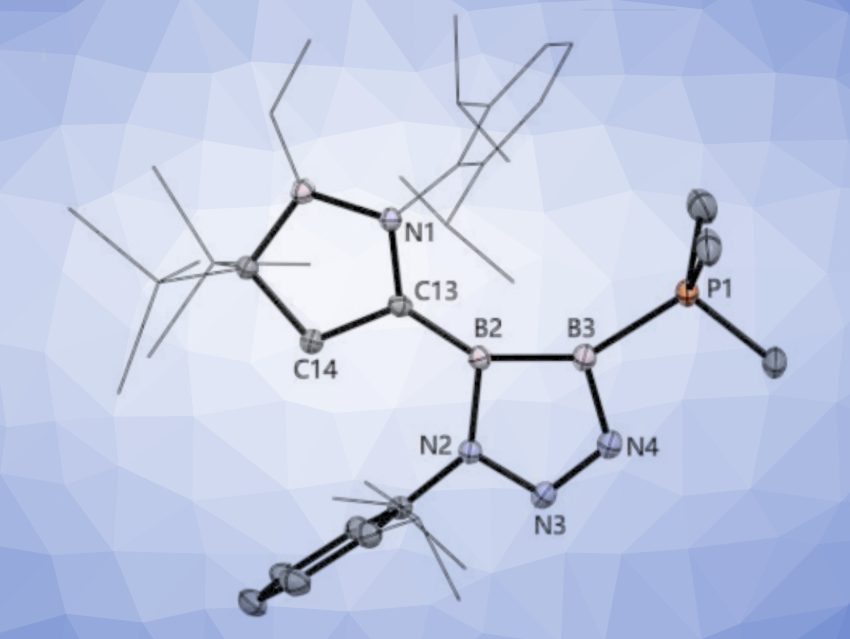
The solid-state structure of the product features a planar five-membered B2N3 ring. Unlike robust organic 1,2,3-triazoles, the diboratriazole spontaneously releases N2 to produce a 2,3-dibora-4-aza-1,3-butenyne derivative. Computational analyses indicate a delocalization of π electrons and a weak but distinct aromatic nature for the 6π system in the diboratriazole derivative. The researchers believe that this type of inorganic click chemistry may allow for the synthesis of various new heterocycles.
- An Inorganic Huisgen Reaction between a 1,2‐Diboraallene and an Azide to Access a Diboratriazole,
Lizhao Zhu, Rei Kinjo,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202207631