Photoredox catalysis has become a useful tool in organic synthesis. Photocatalysts based on Earth-abundant metals could help to improve the sustainability of such processes. However, so far, the performance of such metals cannot easily compete with catalysts based on metals such Ru or Ir. Visible-light induced homolysis (VLIH) of metal-heteroatom bonds can be an interesting alternative to “traditional” photoredox approaches. For example, metal carboxylates can undergo electronic transitions under light irradiation that trigger the homolytic cleavage of the metal-oxygen bond, Thus, there is a range of visible-light-induced decarboxylative transformations based on a carboxylate-to-metal charge transfer process, mainly using cerium, iron, or copper catalysts.
Amandine Guérinot, ESPCI Paris – PSL, CNRS, France, and colleagues have developed an iron-catalyzed, visible-light-driven decarboxylative alkoxyamination (pictured below) that converts carboxylic acids into the corresponding alkoxyamine (pictured on blue) derivatives in the presence of FeBr2 and (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO).
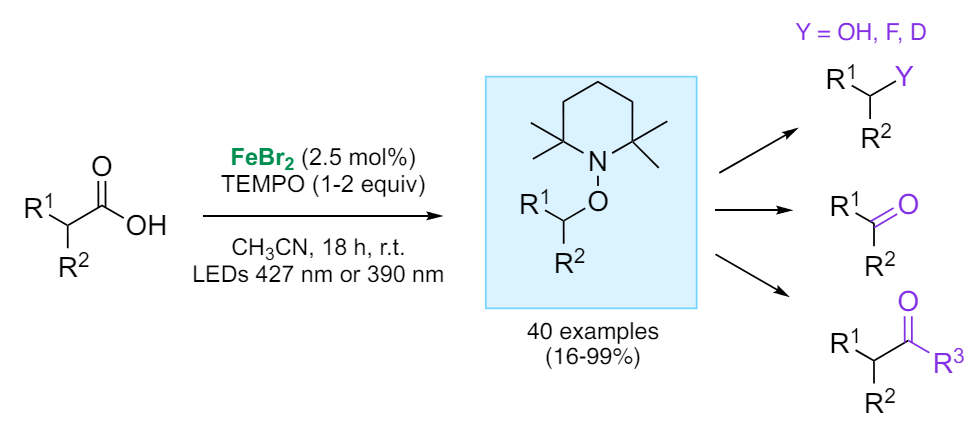
The approach can be used for the conversion of a large range of carboxylic acids (benzylic, non-benzylic, primary, secondary, or tertiary) into the corresponding alkoxyamines in high yields. The reaction proceeds via a carboxyl radical that can rapidly release CO2 and shows a broad functional group tolerance. The products can be further transformed into a variety of useful derivatives (pictured in purple).
- Iron‐Catalyzed, Light‐Driven Decarboxylative Alcoxyamination,
Milan Innocent, Clément Tanguy, Sigrid Gavelle, Thomas Aubineau, Amandine Guérinot,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202401252