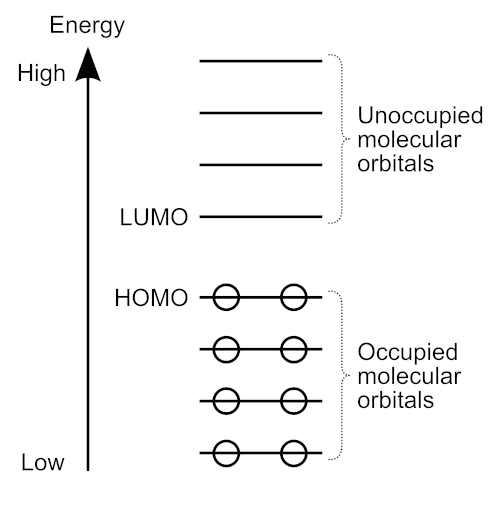
Kenichi Fukui (福井 謙) was a Japanese chemist who performed groundbreaking theoretical work on chemical reactivity and the role of frontier orbitals. Frontier orbitals are those at the “frontier” between occupied and unoccupied orbitals (see diagram below), in particular, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO).
The Nobel Prize in Chemistry 1981 was awarded jointly to Kenichi Fukui and Roald Hoffmann “for their theories, developed independently, concerning the course of chemical reactions.” Fukui was the first person of East Asian descent to be awarded a Nobel Prize in Chemistry.
Fukui’s Life and Career
Kenichi Fukui was born on October 4, 1918, in Oshikuma Nara, Japan [1]. He studied industrial chemistry at Kyoto Imperial University, Japan, starting in 1937. Despite the application-focused field of study, he was interested in basic science and quantum mechanics.
Fukui graduated in 1941 and entered the graduate school in the Department of Fuel Chemistry of the Faculty of Engineering at Kyoto University. In August 1941, Kenichi went to the Fuel Institute of the Japanese Army in Tokyo. In 1943, he became a Lecturer at the Department of Fuel Chemistry at Kyoto University, and in 1944, he was promoted to Associate Professor there. He submitted his doctoral thesis in 1948.
In 1951, Fukui was promoted to Professor in the Department of Fuel Chemistry, a position he held until 1982. He then served as President of the Kyoto Institute of Technology and as President of the Institute for Fundamental Chemistry in Kyoto. Kenichi Fukui passed away on January 9, 1998.
In addition to the Nobel Prize in Chemistry, Fukui received, e.g., the Japan Academy Prize in 1962 for the study of the electronic structure and chemical reactivity of conjugated compounds and was elected a Member of the International Academy of Quantum Molecular Science.
Research
During his graduate studies, Fukui performed experiments on the reactivities of hydrocarbons, a topic that would become important for his later theoretical studies. He observed different reactivities of different hydrocarbons to hexachloroantimony, and was also interested in the reactivities of aromatic hydrocarbons, such as naphthalene and anthracene.
During World War II, Fukui performed experimental research on synthetic fuel chemistry. At the Fuel Institute of the Japanese Army, he worked on the synthesis of hydrocarbons that improve the performance of gasoline.
Frontier Orbital Theory
Fukui’s interests in theoretical physics and quantum mechanics led him to theoretical work on chemical reactions. In 1951, he developed the frontier electron theory [2], proposing that in a chemical reaction, electrons in the “outermost” molecular orbital should play an important role. He calculated the electron density in the HOMO of naphthalene and found that it was largest at the position where a chemical reaction with an electrophile took place. He went on to study other hydrocarbons such as anthracene, pyrene, and perylene.
The frontier electron theory was extended to the frontier orbital theory, which included the role of the LUMO in chemical reactions with nucleophilic reagents [3]. The reactivity with free radicals was determined by the electron densities in both the HOMO and LUMO.
The frontier orbital theory explained not only the reactivities of hydrocarbons, but was also applicable to many other types of compounds. The concept is found, for example, in the Woodward–Hoffmann rules [4] which relate the chemical reactivities of molecules in pericyclic reactions with the natures of their HOMOs and LUMOs—leading to increased attention for the theory and the joint award of the Nobel Prize for Chemistry in 1981.
Intrinsic Reaction Coordinate
In 1970, Fukui defined the route of a chemical reaction, the “intrinsic reaction coordinate” (IRC) [5], as the minimum energy reaction pathway between the transition state of a reaction and its reactants and product. While the idea was simple, Fukui could not find an existing publication on the topic and submitted his manuscript, with the amusing result that the referee report stated that the article had no originality but was worthy of publication. The IRC is widely used in quantum chemical calculations.
Kenichi Fukui is the answer to Guess the Chemist (141).
References
[1] Kenichi Fukui: 4 October 1918 – 9 January 1998,
A. D. Buckingham, H. Nakatsuji,
Biog. Mems. Fell. R. Soc. 2001, 47, 223–237.
https://doi.org/10.1098/rsbm.2001.0013
[2] A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons,
K. Fukui, T. Yonezawa, H. Shingu,
J. Chem. Phys. 1952. 20, 722–725.
https://doi.org/10.1063/1.1700523
[3] Molecular Orbital Theory of Orientation in Aromatic, Heteroaromatic, and Other Conjugated Molecules,
K, Fukui, T. Yonezawa, C. Nagata, H. Shingu,
J. Chem. Phys. 1954, 22, 1433–1442.
https://doi.org/10.1063/1.1740412
[4] Stereochemistry of Electrocyclic Reactions,
R. B. Woodward, R. Hoffmann,
J. Am. Chem. Soc. 1965, 87, 395–397.
https://doi.org/10.1021/ja01080a054
[5] Formulation of the reaction coordinate,
K. Fukui,
J. Phys. Chem. 1970, 74, 4161–4161.
https://doi.org/10.1021/j100717a029
Also of Interest
50th Anniversary: Woodward-Hoffmann Rules,
ChemistryViews 2015.
In 1965, Robert Burns Woodward and Roald Hoffmann explained the stereospecificity of pericyclic reactions