Hydrogenation reactions are important for many synthetic processes. They usually employ H2 gas and transition-metal catalysts based on, e.g., rhodium, palladium, or nickel. Hydride additions to polar double-bond systems are an alternative class of reduction reactions that utilize stoichiometric amounts of main-group metal hydrides, e.g., based on boron, silicon, or aluminium.
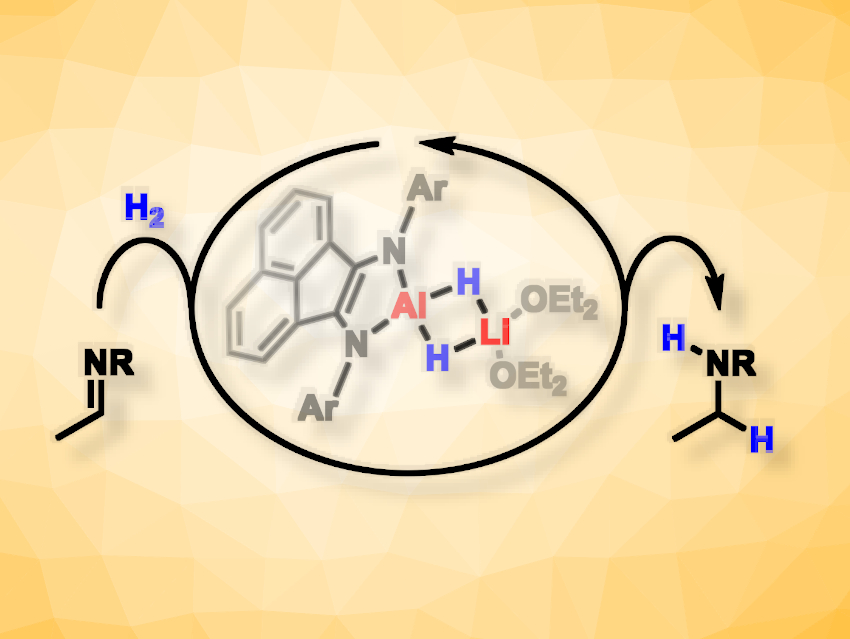
Axel Jacobi Von Wangelin, University of Hamburg, Germany, and colleagues have developed a combination of both of these synthetic concepts, i.e., a main-group metal-catalyzed hydrogenation. The team used the diamido LiAl μ2-dihydride complex [(DippBIAN)Al-(H)2-Li(OEt2)2] (pictured, BIAN = bis(imino)acenaphthene, Dipp = 2,6-diisopropylphenyl) for catalytic hydrogenations of N-alkyl imines, N-aryl imines, and oximes. This catalyst, equipped with a bulky diimine ligand, enabled hydrogenations of various substrates in the presence of H2.
The catalyst was synthesized starting from acenaphthenequinone, which was reacted with 2,6-diisopropylphenylaniline to form a diimine. The diimine was reacted with potassium and then protonated to convert it to an enediamine. The addition of LiAlH4 then gave the desired complex.
Aluminium is Earth-abundant, inexpensive, and less toxic than most heavier metals. This work could be a step toward the replacement of rare and toxic metal catalysts with more abundant main-group elements for improved sustainability.
- BIAN‐Aluminium‐Catalyzed Imine Hydrogenation,
Jennifer Pölker, Dieter Schaarschmidt, Josef Bernauer, Matteo Villa, Axel Jacobi von Wangelin,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200144