Metal salen complexes are interesting species since the salen ligand is known to be redox-active. This means that when the complexes are oxidized, i.e., electrons are removed, it is often not immediately obvious if these electrons come from the ligand or the metal. In fact, in catalyst design, this can be used to one’s advantage. Ligands can act as electron reservoirs, enabling complexes to increase their oxidizing equivalents. However, understanding where redox events are centered is crucial when constructing and tuning catalytic cycles.
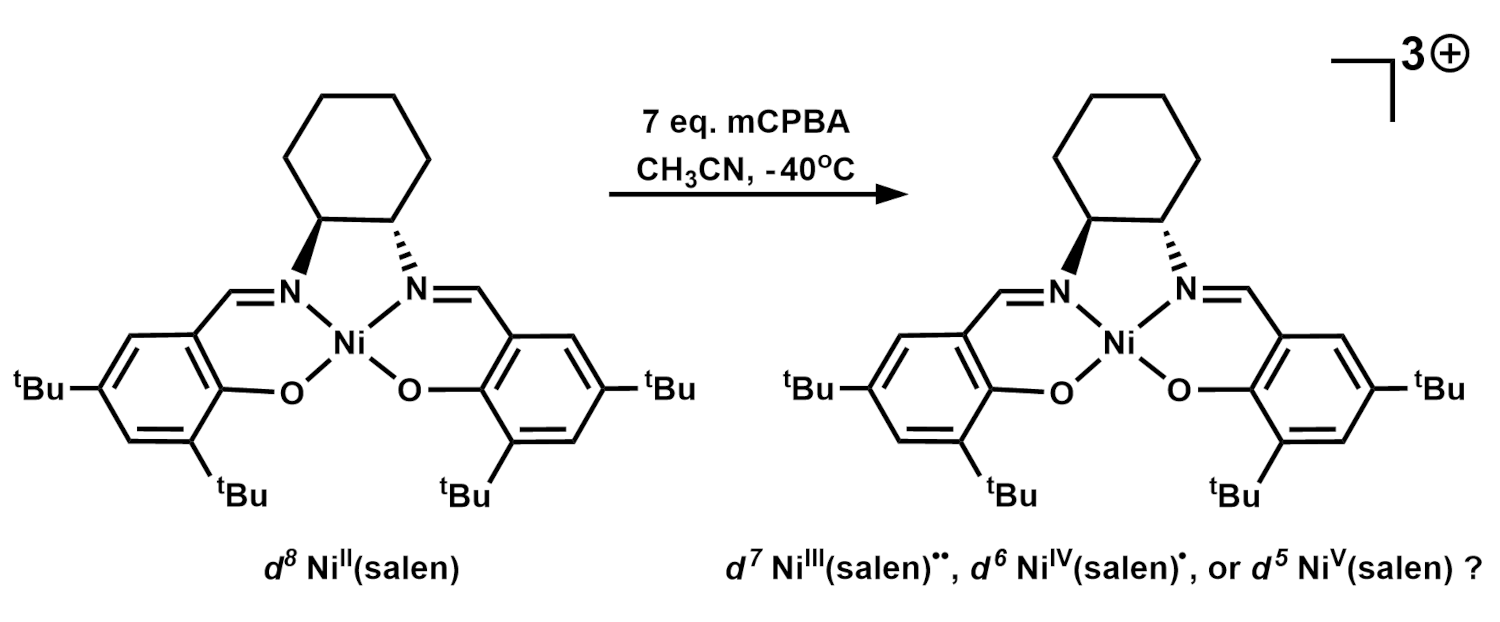
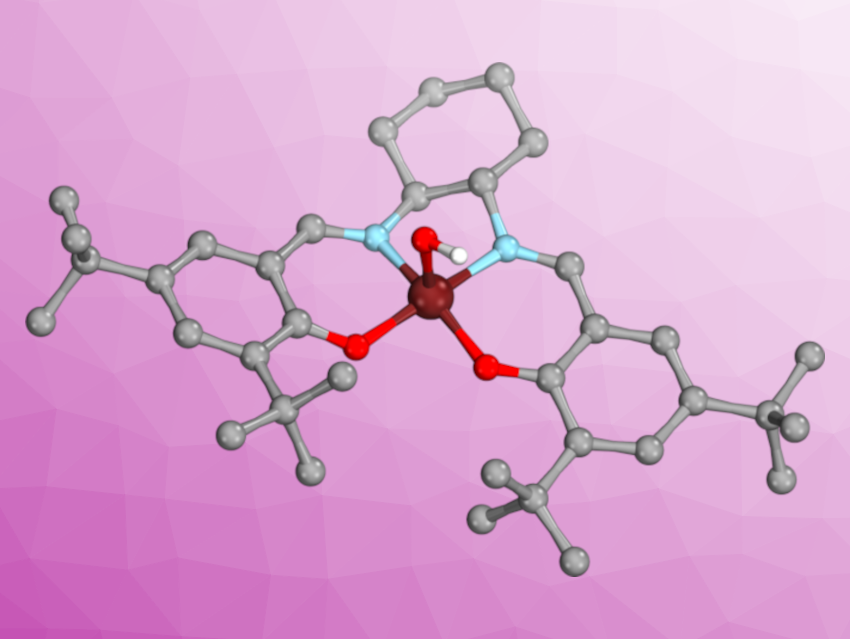
Johannes Klein, University of Groningen, The Netherlands, Apparao Draksharapu, Indian Institute of Technology Kanpur, India, and colleagues have investigated the triply oxidized nickel salen complex shown above on the right, which exhibits hydrogen atom abstraction and oxygen atom transfer reactivity, using spectroscopy and density functional theory (DFT) calculations. The complex is an unprecedented example of redox activity that is difficult to pinpoint, since nickel can access a wide range of oxidations states (–I to +IV), and the salen ligand can be redox-neutral (salen), singly oxidized (salen)●, or doubly oxidized (salen)●●.
Oxidation at Both Ligand and Metal
The team triply oxidized the precursor complex NiII(salen) (pictured above on the left, salen = N,N‘-bis(3,5-di-tert-butyl-salicylidene)-1,2-cyclohexane-(1R,2R)-diamine) using meta-chloroperoxybenzoic acid (mCPBA) in acetonitrile (CH3CN) at –40 °C. Electron paramagnetic (EPR) spectroscopy, which detects unpaired electrons, showed signals for the oxidized species that were assigned to NiIII(salen)●●, with three unpaired electrons antiferromagnetically coupled (↑↓↑ or ↓↑↑). Titrations with ferrocene confirmed the presence of three oxidizing equivalents. These data, as well as electrochemistry experiments, showed that one of the electrons came from the metal, giving nickel(III), while the other two were removed from the ligand, giving (salen)●●.
The +III oxidation state of the nickel center was easily identified in DFT calculations by its intrinsic d7 configuration, which was found to engage in dative bonding with the doubly oxidized ligand. However, this was only the case when the complex was modeled with an axial ligand, e.g., hydroxide (OH–) or acetonitrile, demonstrating the axial ligands’ ability to alter the electronic structure, and therefore, the importance of carefully considering this aspect in computational studies.
While high-valent nickel complexes with a metal oxidation state greater than +III are difficult to access, this work shows how the reactivity of nickel(III) complexes can be enhanced by utilizing redox-active ligands as electron reservoirs.
- Formation and Reactivity of a Fleeting Ni(III) Bisphenoxyl Diradical Species,
Ayushi Awasthi, Isaac F. Leach, Silène Engbers, Rakesh Kumar, Raju Eerlapally, Sikha Gupta, Johannes E. M. N. Klein, Apparao Draksharapu,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202211345