Huan-Ming Huang and colleagues, ShanghaiTech University, China, have demonstrated two visible-light-driven methods to construct borylated strained ring systems, key motifs in drug and materials development. The team uses energy transfer catalysis to enable a rare type of photorearrangement involving boron.
In the first method, the researchers designed a substrate featuring a carbon–boron σ-bond and a nearby carbon–carbon π-bond, separated by a single sp³ carbon. Under visible light and energy transfer catalysis, this arrangement undergoes what they term a “π, σ-methane rearrangement.” Activation generates a diradical species, triggering a 1,4-boron shift and smoothly forming borylated cyclopropanes.
When a weak C–H bond is present, energy transfer similarly yields a diradical, but this time it undergoes 1,5-hydrogen atom transfer (HAT) followed by cyclization, leading to borylated cyclobutanes.
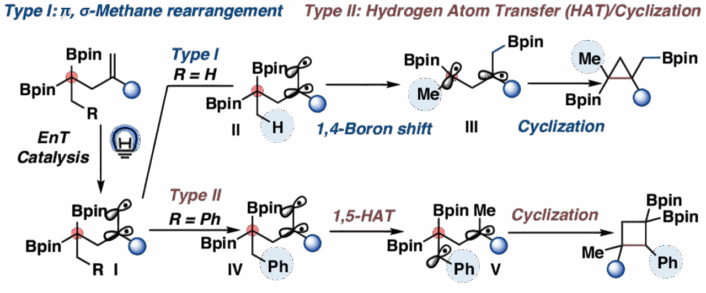
HBpin = pinacolborane
Together, these two complementary pathways show how the combination of suitable visible-light energy transfer catalysis and substrate design can efficiently generate structurally complex, borylated strained rings under mild conditions. The catalytic platform permits a broad substrate scope and good functional group toleration. According to the researchers, this provides chemists with powerful new tools for building boron-rich frameworks.
- Borylated strain rings synthesis via photorearrangements enabled by energy transfer catalysis,
Shu-Sheng Chen, Yu Zheng, Zhi-Xi Xing, Huan-Ming Huang,
Nature Communications 2025, 16, 3724.
https://doi.org/10.1038/s41467-025-58353-w