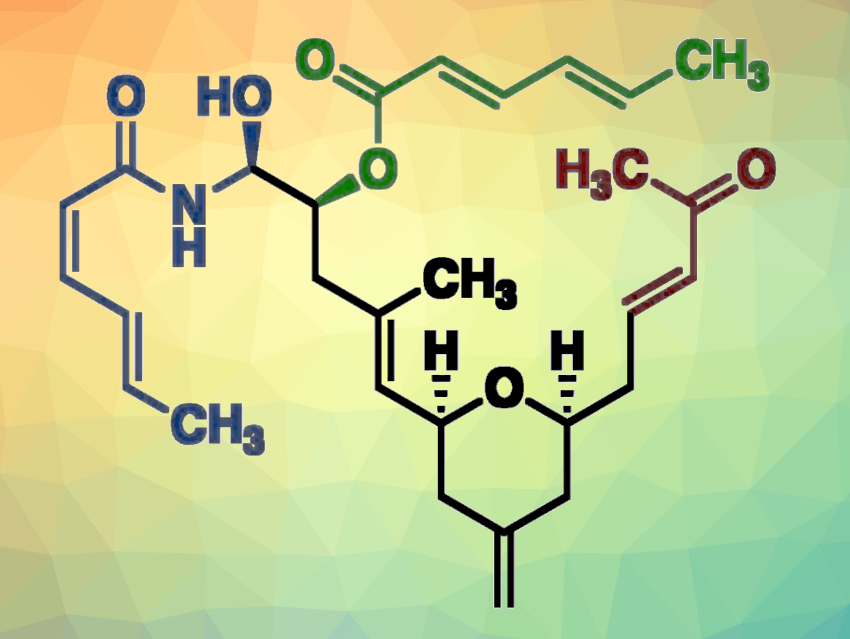
(−)-Zampanolide is a natural product that was isolated from a marine sponge. Its structure features a highly unsaturated 20-membered macrolactone and an unusually stable N-acyl hemiaminal side chain. The compound has shown activity against different cancer cell lines. The conformational preferences and flexibility of macrocycles such as (−)-zampanolide can affect their biological activity.
Richard E. Taylor, University of Notre Dame, Notre Dame, IN, USA, and colleagues have developed a linear variant of (−)-zampanolide (pictured) and found that it retains high activity towards cancer cell lines. The linear analogue was synthesized starting from (R)-aspartic acid, which was converted to an enantiomerically pure alcohol. The alcohol was converted to a β-alcoxyacrylate intermediate, followed by an intramolecular radical cyclization, an ester reduction, and a iodination and subsequent elimination to obtain a terminal olefin. Cross-metathesis with methacrolein then gave a conjugate dienonate. Further steps include a diastereoselective Sharpless epoxidation to give an epoxy-alcohol, a hydride epoxide opening, and the installation of an exo-methylene group using a Wittig olefination.
The obtained intermediate was further functionalized, and the α,β-unsaturated ketone unit (pictured in red) was introduced using a Horner-Wadsworth-Emmons (HWE) olefination with diethyl (2-oxopropyl)phosphonate. A Yamaguchi esterification with sorbic acid was used to install the C19 acyl group, and the hemiaminal side chain was introduced using a diastereoselective aza-aldol reaction. Overall, the team obtained a single diastereomer of the desired linear zampanolide analogue in 25 steps as the longest linear sequence starting from the enantiomerically pure alcohol.
The product lacks the constraints of the natural product’s macrocyclic ring but retains the potent cytotoxic activity of the parent compound. According to the researchers, this demonstrates that increased overall conformational flexibility is not necessarily detrimental to protein binding affinity and biological activity. The synthesized linear analogue could be further diversified to find other biologically active derivatives.
- Linear (−)‐Zampanolide: Flexibility in Conformation–Activity Relationships,
Christian A. Umaña, Jeffrey L. Henry, Claire T. Saltzman, Dan L. Sackett, Lisa M. Jenkins, Richard E. Taylor,
ChemMedChem 2023.
https://doi.org/10.1002/cmdc.202300292