Trialkylaluminum reagents can be useful, e.g., in organic synthesis or polymer synthesis. They can be obtained, for example, by alkene hydroalumination. Noncatalytic reactions of terminal alkenes and aluminum hydrides can be used for this, but generally have a limited substrate scope and low selectivity. Lewis-acid or transition-metal catalysts can improve the selectivity and allow reactions under milder conditions. This type of reaction generally gives anti-Markovnikov hydroalumination products.
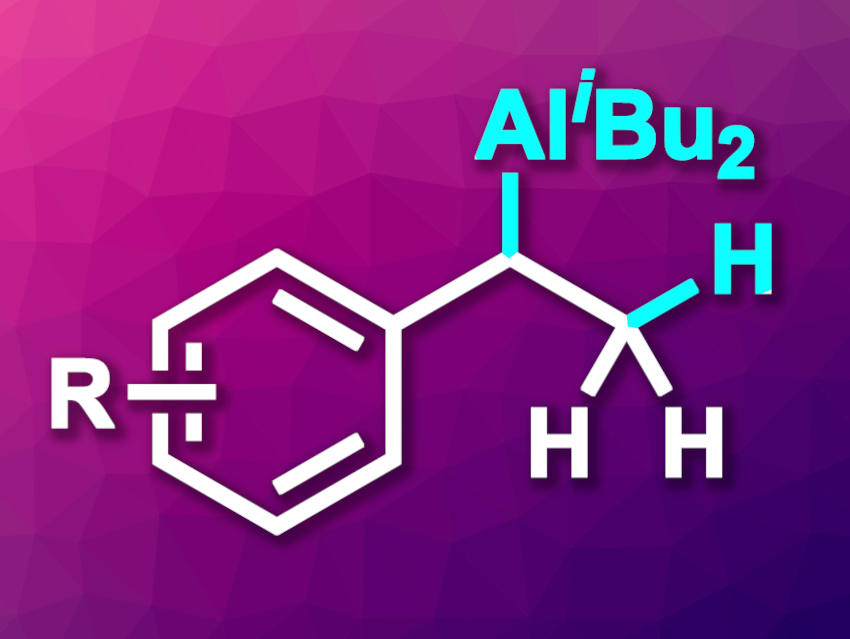
Shou-Fei Zhu, Nankai University, Tianjin, China, and colleagues have developed an iron-catalyzed hydroalumination of aromatic terminal alkenes that gives the Markovnikov products (example structure pictured). The team reacted a variety of terminal alkenes with a (hetero)aromatic substituent with diisobutylaluminium hydride (DIBAL-H) as an aluminum hydride reagent in the presence of an iron complex with a 2,9-bis(3,5-di-tert-butylphenyl)phenanthroline ligand as the catalyst. The reactions were performed at 30 °C in tetrahydrofuran (THF).
The desired hydroalumination products were obtained in the form of solvent complexes and can be subjected to ligand exchange using 4-dimethylaminopyridine (DMAP). Yields were moderate to excellent, and the products can be further transformed to introduce several different types of functional groups. The regioselectivity achieved by this iron-catalyzed method complements existing methods.
- Iron-Catalyzed Alkene Hydroalumination,
Wen-Tao Li, Qiao Zhang, Meng-Yang Hu, Shou-Fei Zhu,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02044