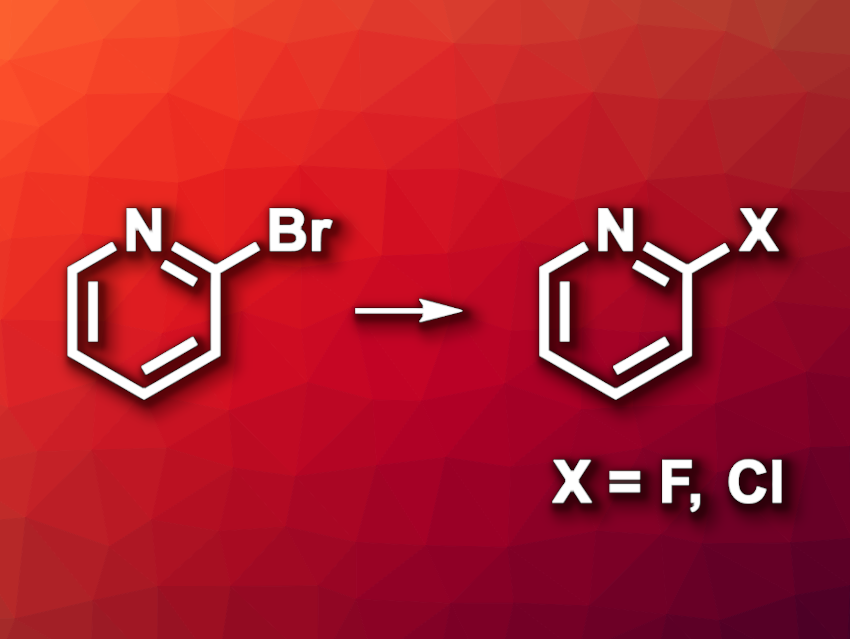
Metal–organic frameworks (MOFs) are crystalline, porous materials composed of metal centers and organic linkers. They have applications, e.g., in gas separation or catalysis. Halogen-functionalized compounds are important products in organic synthesis. 2-Haloheteroarenes, for example, are commonly used in medicinal chemistry. Still, the late-stage introduction of halogens into bioactive compounds can be challenging, in particular, when using low-cost metal halide salts as reactants.
Phillip J. Milner, Cornell University, Ithaca, NY, USA, and colleagues have found that a Co(III)-based MOF with terminal Co(III) halide sites can serve as a heterogeneous catalyst for the introduction of chlorine or fluorine into (hetero)aryl bromides (example reactions pictured). The team synthesized the MOFs Co2X2Cl2(btdd) (X = F, Cl, Br, I; btdd2– = bis(1H-1,2,3-triazolo[4,5-b],[4′,5′-i])dibenzo[1,4]dioxin)) starting from H2btdd and CoCl2·6H2O, which gave Co2Cl2(btdd). The different halogenated analogues were then obtained via one-electron oxidation with electrophilic halogen sources.
The researchers used Co2Cl4(btdd) as a catalyst for halogen exchange reactions with (hetero)aryl bromides, employing KCl as a low-cost chloride source. The desired products were obtained in mostly moderate to high yields. Similarly, they performed Co2Br2Cl2(btdd)-catalyzed fluorinations of (hetero)aryl bromides using CsF as a fluoride source. The MOF catalysts can be used for gram-scale reactions and recycled/reused, as well as employed in continuous-flow reactions.
- Cobalt(III) Halide Metal–Organic Frameworks Drive Catalytic Halogen Exchange,
Tyler J. Azbell, Phillip J. Milner,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c13872