Microdroplets can act like tiny chemical reactors, and their special physicochemical properties can accelerate some reactions, sometimes by several orders of magnitude. They can also remove a need for catalysts or change the product distribution. Knowing how exactly microdroplets influence reactions would be useful for their application. However, this has not been fully understood so far. Factors that could have an influence are, for example, partial solvation of the reactants or transition states, the pH in the droplets, electric fields, concentration changes to due evaporation, etc.
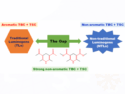
Xinxing Zhang, Nankai University, Tianjin, China, Jing Xie, Beijing Institute of Technology, China, and colleagues have investigated how microdroplets influence a model aza-Michael addition reaction between methylamine and acrylamide. The team used quantum chemistry calculations to study the effects of partial solvation, pH values, and interfacial electric fields (IEFs) in methanol or water microdroplets. They also performed the reaction experimentally, both in a spray of microdroplets and in the bulk. In water microdroplets and methanol microdroplets, the yield of the reaction was 84 % and 57 %, respectively, under the same conditions. Compared with the bulk, water microdroplets can accelerate the reaction by a factor of over 107.
The researchers used density functional theory (DFT) calculations to determine the structures of the chemical species involved and more accurate ab–initio calculations to determine the energies of the optimized structures. To study the effects of IEFs, they simulated electric fields of different strengths that are consistent with those at the microdroplet interface. To account for the pH values, the team simulated protonated species, and for the effects of partial solvation, they performed calculations with different numbers of solvent molecules included.
The researchers found that the reaction acceleration is based on several factors. The acidic environment at the microdroplet surface is key, lowering the activation barrier by ca. 9 kcal mol–1. This influence of the acidity is also consistent with the higher yield in water compared with methanol. The IEF can also decrease the barrier by about 2 kcal mol–1. According to the work, partial solvation only has a negligible effect. The team points out that the studied reaction is influenced by the pH value and the results might not be transferable to other reactions with different mechanisms.
- Deciphering the Microdroplet Acceleration Factors of Aza-Michael Addition Reactions,
Zhexuan Song, Chenghui Zhu, Ke Gong, Ruijing Wang, Jianze Zhang, Supin Zhao, Zesheng Li, Xinxing Zhang, Jing Xie,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c02312