Frédéric Avenier, Université Paris-Saclay, France, discusses a recent paper published with coauthors in ChemistryEurope, where they describe a simple iron-based method to attach an NH group to sulfides using an air-stable catalyst that works at room temperature. Their method efficiently converts diverse sulfides into sulfilimines, enabling late-stage pharmaceutical modification.
What did you do?
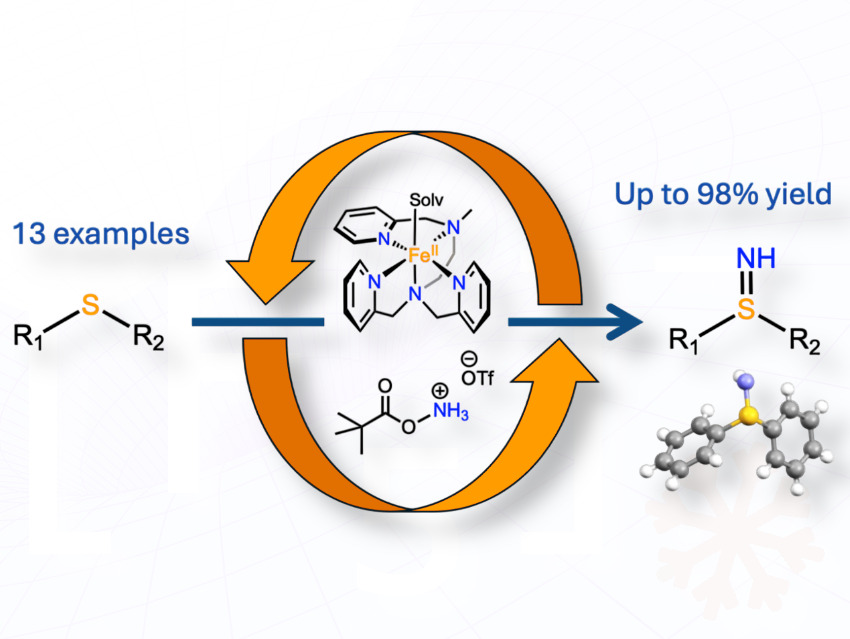
We demonstrated that the bioinspired mononuclear iron(II) complex [Fe(II)MeTPEN](OTf)₂ catalyzes nitrene transfer reactions—specifically the sulfilimination (>S=NH) of thioethers—in good yields at room temperature under aerobic conditions.
[Editor’s note: TPEN is an acronym for N,N,N’,N’-tetrakis(2-pyridylmethyl)ethylenediamine; OTf stands for trifluoromethanesulfonate (–OSO₂CF₃), also called triflate]
Why are you interested in this?
Catalytic amination is attracting continuous attention due to the importance of the nitrogen atom in medicinal chemistry and other industrial applications. According to the FDA, the U.S. Food and Drug Administration, it is estimated that 80 % of therapeutic molecules contain at least one nitrogen atom, which is often unprotected.
What is new and cool about your work?
Unlike previous methods requiring protected nitrogen sources, this approach uses an air-stable iron catalyst with an unprotected nitrene donor at room temperature in the presence of oxygen.
Furthermore, as a bio-inspired system, it also uses an earth abundant ion, combining sustainability and low cost.
The method works on a variety of sulfides, including complex molecules like penicillin derivatives, showing practical use in medicinal chemistry, and it produces sulfilimines efficiently with minimal side reactions, making it a potentially reliable synthetic tool.
What are your key findings?
We have established mild conditions for the preparation of various aminated substrates, including the late-stage functionalization of biomolecules such as biotin-sulfilimine and therapeutic molecules such as penicillin G sulfilimine, aiming to tune their antibiotic activity and potentially extend their use as antiviral agents.
Can you say something about the mechanisms involved?
We found that the [Fe(II)MeTPEN] complex activates the hydroxylamine derivative (PivONH₃⁺) to generate a reactive nitrene species which still needs to be characterized. It is then transferred directly to the sulfide substrate to form the sulfilimine, without requiring a protected nitrogen source. The reaction proceeds at room temperature under air, suggesting that the iron catalyst stabilizes the reactive intermediates and prevents side reactions.
We are also very interested in comparing the mechanism of this nitrene transfer reaction with the well-established iron-catalyzed oxene transfer chemistry, which is inspired by natural iron oxygenases, to understand similarities in how the iron center mediates atom transfer.
What specific applications do you imagine?
The first potential application is the late-stage functionalization of molecules of interest, and we are now working on the chiral form of the iron catalyst to potentially produce enantiomerically pure sulfilimines.
The long-term vision of the project will be to evolve the catalyst structure towards more reactive intermediates with the aim to catalyze other nitrene transfer reactions, such as aziridination or C–H amination reactions.
What part of your work was the most challenging?
The most challenging part of our work is obtaining unprotected aminated products under aerobic conditions, as they are more prone to oxidation and less stable during purification.
Thank you very much for sharing these insights and all the best for your future work!
The paper they talked about:
- NH-Sulfilimine Synthesis Catalyzed by Nonheme Iron(II) Complex,
Léa Tesson, Jean-Pierre Mahy, Frédéric Avenier,
ChemistryEurope 2025.
https://doi.org/10.1002/ceur.202500395

Frédéric Avenier is a professor at the Institut de Chimie Moléculaire et des Matériaux d’Orsay (UMR CNRS 8182), Université Paris-Saclay, France.
Also of Interest