Organic compounds with sulfur atoms as stereogenic centers are useful, e.g., in pharmaceutical chemistry. In this context, cyclic chiral sulfur compounds have received less attention than their acyclic counterparts. New approaches to the catalytic synthesis of sulfur-stereogenic chiral heterocycles would be helpful, e.g., for drug discovery.
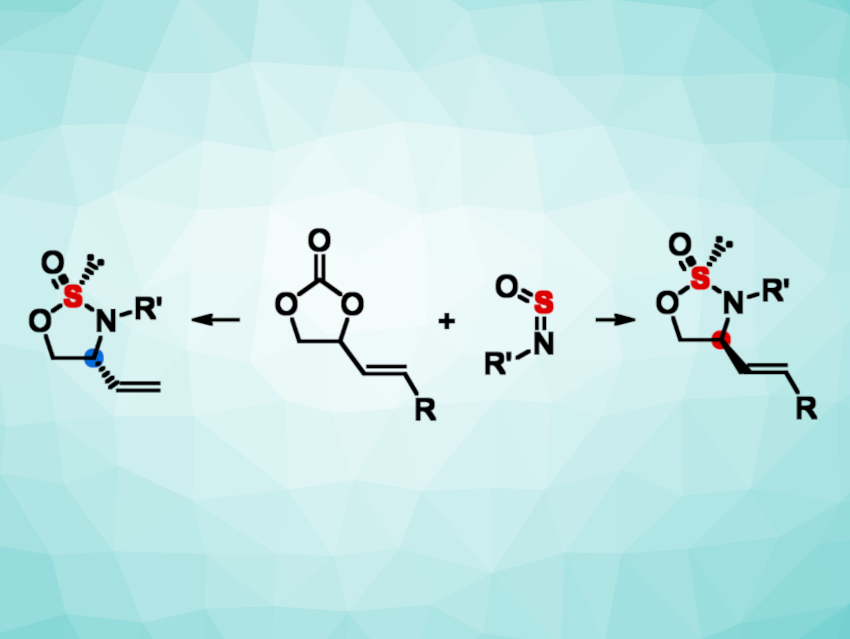
Yin-Long Guo, Shanghai Institute of Organic Chemistry, University of the Chinese Academy of Sciences, Liang-Qiu Lu, Central China Normal University, Wuhan, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, and Henan Normal University, Xinxiang, China, and colleagues have developed a modular platform that provides access to chiral thiooxazolidinones with S(IV)-stereogenic centers. The approach involves palladium-catalyzed asymmetric [3+2] annulations of vinylethylene carbonates and sulfinylanilines (general reaction pictured).
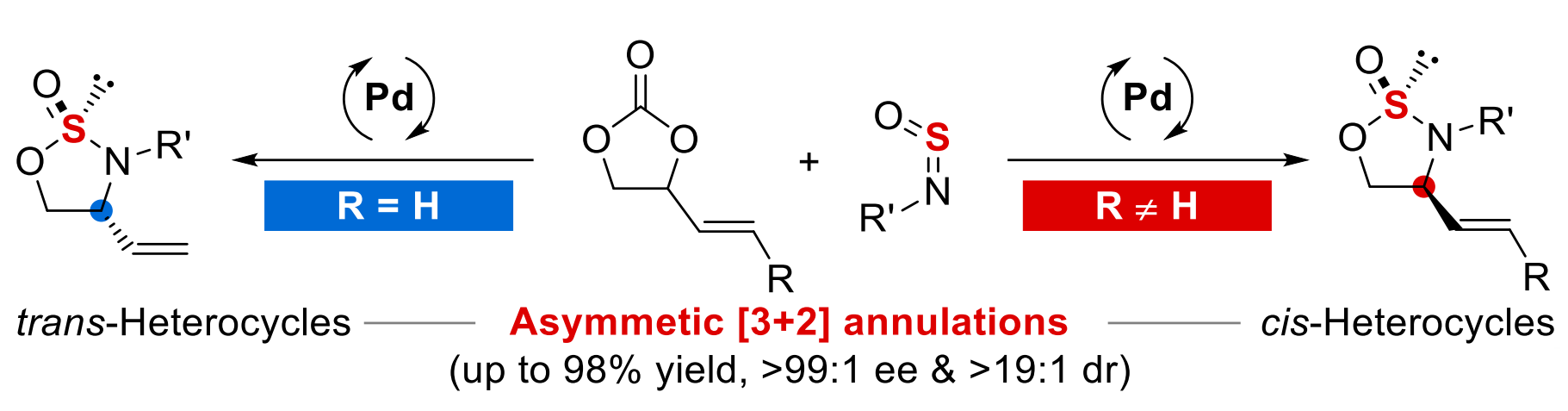
The team used Pd2(dba)3·CHCl3 as a catalyst together with a chiral urea-containing phosphine ligand and CH2Cl2 as a solvent to react different vinylethylene carbonates (R = H, see above) with various N-aryl-substituted sulfinylamines at 13 °C. They obtained the desired products in a trans-selective manner (pictured above on the left).
For substituted vinylethylene carbonates, (R ≠ H, see above), they used Pd(PPh3)4 as a catalyst together with a different chiral urea-containing phosphine ligand and tetrahydrofuran (THF) as the solvent to perform the reactions at 15 °C. This led to the cis-selective formation of chiral thiooxazolidinones (pictured above on the right). Overall, the work provides a modular path to useful chiral heterocycles using readily available starting materials that provides high enantio- and diastereoselectivity.
- Synthesis of S(IV)‐Stereogenic Chiral Thio‐Oxazolidinones via Palladium‐Catalyzed Asymmetric [3+2] Annulations,
Bao-Cheng Wang, Fang Hu, Jiahui Bai, Fen-Ya Xiong, Peng Chen, Jianye Li, Ying Tan, Yin-Long Guo, Wen-Jing Xiao, Liang-Qiu Lu,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202319728