Carbaporphyrins are porphyrin analogues in which one or more nitrogen atoms in the core are replaced by carbon atoms. Polycyclic aromatic hydrocarbons (PAHs), such as naphthalene or anthracene, can be used to create expanded carbaporphyrins. So far, large PAHs like nanographenes had not been used to form such carbaporphyrins.
Juwon Oh, Soonchunhyang University, Asan, Republic of Korea, Dongho Kim, Yonsei University, Seoul, Republic of Korea, Jonathan L. Sessler, The University of Texas at Austin, USA, Xian-Sheng Ke, Beijing Normal University, China, and colleagues have synthesized a carbaporphyrin that is fused to nanographenes, as well as the analogous BF2 complex.
The team started from a tetraaryl cyclopentadienone, which was subjected to a Diels–Alder reaction with 1,2-bis(4-(tert-butyl)phenyl) ethyne to give a hexaaryl-functionalized benzene derivative. This intermediate was then treated with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) in the presence of trifluoromethanesulfonic acid to give a fused product. This product was converted to a bicarbinol, and a condensation with pentafluorophenyl-substituted dipyrromethane gave the desired carbaporphyrin. The BF2 complex could be obtained by treatment of this product with an excess of BF3·OEt2 in the presence of N,N-diisopropylethylamine (DIPEA).
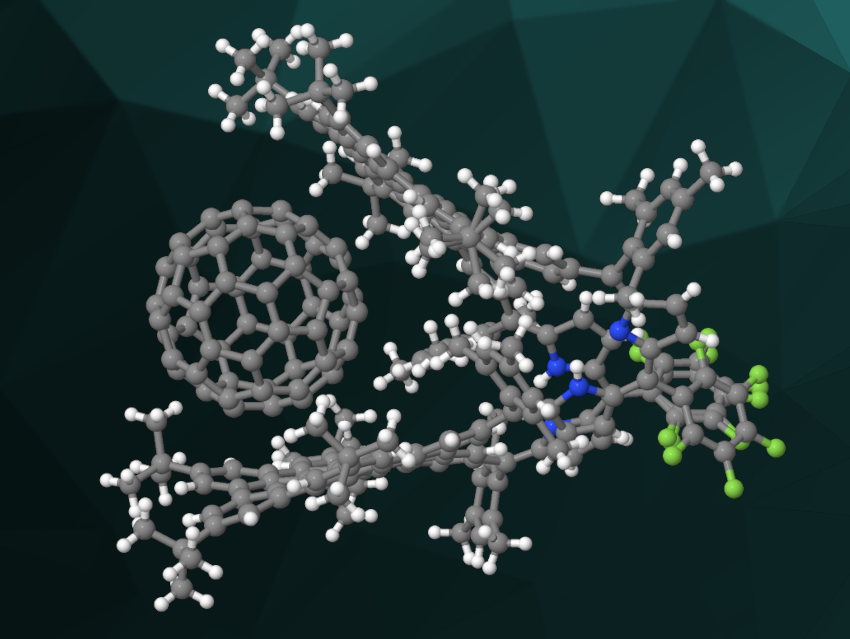
Both products have tweezer-like conformations. The team studied the interactions of these molecular tweezers with the fullerene C60. They found that both compounds bind C60 in a 1:1 complex (example pictured). According to the researchers, the work represents the first example of a nanographene building block being incorporated into the core of a porphyrinic framework.
- Nanographene-Fused Expanded Carbaporphyrin Tweezers,
Haodan He, Yu Jin Lee, Zhaohui Zong, Ningchao Liu, Vincent M. Lynch, Jinseok Kim, Juwon Oh, Dongho Kim, Jonathan L. Sessler, Xian-Sheng Ke,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c10122