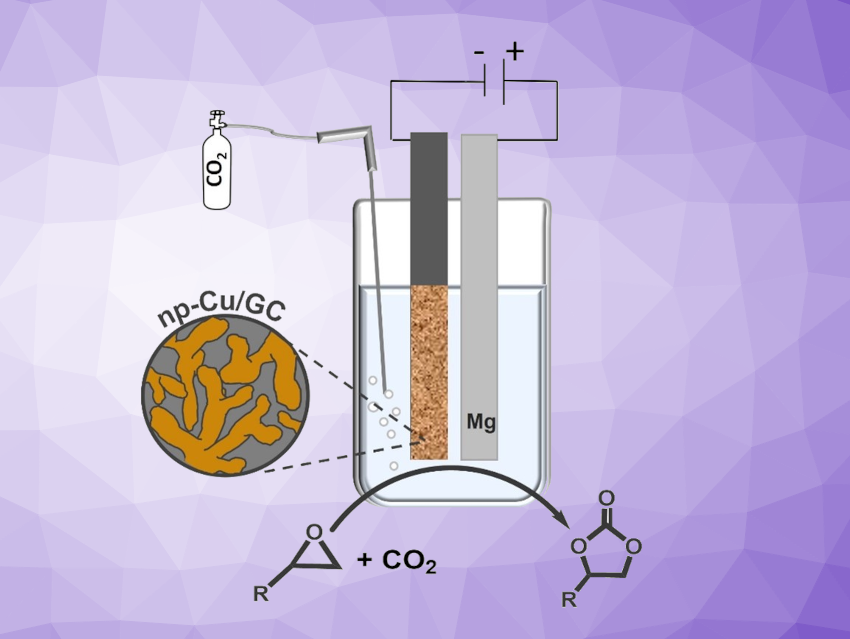
Cyclic carbonates are important chemicals used, e.g., as electrolytes in lithium-ion batteries, lubricants, and polycarbonate precursors. They are often produced by the catalytic coupling of CO2 with epoxides at high temperatures and CO2 pressures. Electrosynthesis could offer a more environmentally friendly and energy-efficient synthetic approach. For the formation of cyclic carbonates, CO2 activation is required, involving the electro-reduction of CO2 to the CO2• – radical anion. Copper cathode materials can be useful for this reaction, and nanostructured materials with their large surface areas might be particularly promising.
Mehtap Oezaslan, Carl von Ossietzky University of Oldenburg, Germany, and Technische Universität Braunschweig, Germany, and colleagues have used highly active and robust nanoporous Cu cathode materials (np-Cu) for the electrosynthesis of cyclic carbonates from CO2 and epoxides under mild conditions (simplified setup pictured). The team prepared np-Cu that was drop-cast onto a glassy carbon substrate (np-Cu/GC). The resulting np-Cu-coated GC plates were used as cathodes for the electrolysis.
The researchers found that although the nanostructuring of np-Cu is beneficial to the performance for CO2 activation, similar yields were achieved on polycrystalline Cu. Thus, the work shows that CO2 activation is not the rate-limiting step for this reaction, but rather the ring-closure of the final intermediate. The team plans to explore the utilization of np-Cu electrodes for other reactions, such as the electrocarboxylation of dienes or α,β-unsaturated esters with CO2 in the future.
- Nanoporous Copper for the Electrosynthesis of Cyclic Carbonates from CO2 and Epoxides,
Sawsan Ibrahim, Daniel Crespo, Sonja Blaseio, Andre Hockmann, Gerhard Hilt, Mehtap Oezaslan,
ChemElectroChem 2024.
https://doi.org/10.1002/celc.202400098